Pharmaceutical industry sponsorship and research outcome and quality: systematic review
- PMID: 12775614
- PMCID: PMC156458
- DOI: 10.1136/bmj.326.7400.1167
Pharmaceutical industry sponsorship and research outcome and quality: systematic review
Abstract
Objective: To investigate whether funding of drug studies by the pharmaceutical industry is associated with outcomes that are favourable to the funder and whether the methods of trials funded by pharmaceutical companies differ from the methods in trials with other sources of support.
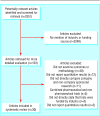
Methods: Medline (January 1966 to December 2002) and Embase (January 1980 to December 2002) searches were supplemented with material identified in the references and in the authors' personal files. Data were independently abstracted by three of the authors and disagreements were resolved by consensus.
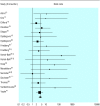
Results: 30 studies were included. Research funded by drug companies was less likely to be published than research funded by other sources. Studies sponsored by pharmaceutical companies were more likely to have outcomes favouring the sponsor than were studies with other sponsors (odds ratio 4.05; 95% confidence interval 2.98 to 5.51; 18 comparisons). None of the 13 studies that analysed methods reported that studies funded by industry was of poorer quality.
Conclusion: Systematic bias favours products which are made by the company funding the research. Explanations include the selection of an inappropriate comparator to the product being investigated and publication bias.
Figures
References
-
- Wyatt J. Use and sources of medical knowledge. Lancet 1991;338: 1368-73. - PubMed
-
- Anderson JJ, Felson DT, Meenan RF. Secular changes in published clinical trials of second-line agents in rheumatoid arthritis. Arthritis Rheum 1991;34: 1304-9. - PubMed
-
- Dorman PJ, Counsell C, Sandercock P. Reports of randomized trials in acute stroke, 1955 to 1995: what proportions were commercially sponsored? Stroke 1999;30: 1995-8. - PubMed
-
- Pharmaceutical Research and Manufacturers of America. 2001 industry profile. Washington, DC: PhRMA, 2003. www.phrma.org/publications/publications/profile02/index.cfm (accessed 6 May 2003).
-
- Anis AH, Gagnon Y. Using economic evaluations to make formulary coverage decisions: so much for guidelines. Pharmacoeconomics 2000;18: 55-62. - PubMed
