Evidence b(i)ased medicine--selective reporting from studies sponsored by pharmaceutical industry: review of studies in new drug applications
- PMID: 12775615
- PMCID: PMC156459
- DOI: 10.1136/bmj.326.7400.1171
Evidence b(i)ased medicine--selective reporting from studies sponsored by pharmaceutical industry: review of studies in new drug applications
Abstract
Objectives: To investigate the relative impact on publication bias caused by multiple publication, selective publication, and selective reporting in studies sponsored by pharmaceutical companies.
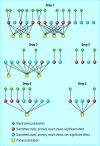
Design: 42 placebo controlled studies of five selective serotonin reuptake inhibitors submitted to the Swedish drug regulatory authority as a basis for marketing approval for treating major depression were compared with the studies actually published (between 1983 and 1999).
Results: Multiple publication: 21 studies contributed to at least two publications each, and three studies contributed to five publications. Selective publication: studies showing significant effects of drug were published as stand alone publications more often than studies with non-significant results. Selective reporting: many publications ignored the results of intention to treat analyses and reported the more favourable per protocol analyses only.
Conclusions: The degree of multiple publication, selective publication, and selective reporting differed between products. Thus, any attempt to recommend a specific selective serotonin reuptake inhibitor from the publicly available data only is likely to be based on biased evidence.
Figures


References
-
- Dickersin K. The existence of publication bias and risk factors for its occurrence. JAMA 1990;263: 1385-9. - PubMed
-
- Dickersin K, Chalmers I. Under-reporting research is scientific misconduct. JAMA 1990;263: 1405-8. - PubMed
-
- Gøtzsche PC. Multiple publication of reports of drug trials. Eur J Clin Pharmacol 1989;36: 429-32. - PubMed
-
- Davidoff F, DeAngelis C, Drazen J, Hoet J, Højgaard L, Horton R, et al. Sponsorship, authorship, and accountability [commentary]. Lancet 2001;358: 854-6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
