Purification and properties of an intracellular 3-hydroxybutyrate-oligomer hydrolase (PhaZ2) in Ralstonia eutropha H16 and its identification as a novel intracellular poly(3-hydroxybutyrate) depolymerase
- PMID: 12775684
- PMCID: PMC156217
- DOI: 10.1128/JB.185.12.3485-3490.2003
Purification and properties of an intracellular 3-hydroxybutyrate-oligomer hydrolase (PhaZ2) in Ralstonia eutropha H16 and its identification as a novel intracellular poly(3-hydroxybutyrate) depolymerase
Abstract
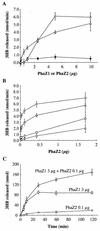
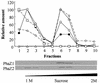
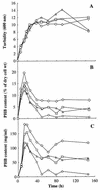
An intracellular 3-hydroxybutyrate (3HB)-oligomer hydrolase (PhaZ2(Reu)) of Ralstonia eutropha was purified from Escherichia coli harboring a plasmid containing phaZ2(Reu). The purified enzyme hydrolyzed linear and cyclic 3HB-oligomers. Although it did not degrade crystalline poly(3-hydroxybutyrate) (PHB), the purified enzyme degraded artificial amorphous PHB at a rate similar to that of the previously identified intracellular PHB (iPHB) depolymerase (PhaZ1(Reu)). The enzyme appeared to be an endo-type hydrolase, since it actively hydrolyzed cyclic 3HB-oligomers. However, it degraded various linear 3HB-oligomers and amorphous PHB in the fashion of an exo-type hydrolase, releasing one monomer unit at a time. PhaZ2 was found to bind to PHB inclusion bodies and as a soluble enzyme to cell-free supernatant fractions in R. eutropha; in contrast, PhaZ1 bound exclusively to the inclusion bodies. When R. eutropha H16 was cultivated in a nutrient-rich medium, the transient deposition of PHB was observed: the content of PHB was maximized in the log growth phase (12 h, ca. 14% PHB of dry cell weight) and decreased to a very low level in the stationary phase (ca. 1% of dry cell weight). In each phaZ1-null mutant and phaZ2-null mutant, the PHB content in the cell increased to ca. 5% in the stationary phase. A double mutant lacking both phaZ1 and phaZ2 showed increased PHB content in the log phase (ca. 20%) and also an elevated PHB level (ca. 8%) in the stationary phase. These results indicate that PhaZ2 is a novel iPHB depolymerase, which participates in the mobilization of PHB in R. eutropha along with PhaZ1.
Figures
References
-
- Doi, Y., A. Segawa, Y. Kawaguchi, and M. Kunioka. 1990. Cyclic nature of poly(3-hydroxyalkanoate) metabolism in Alcaligenes eutrophus. FEMS Microbiol. Lett. 67:165-170. - PubMed
-
- Gaudet, G., E. Forano, G. Dauphin, and A.-M. Delort. 1992. Futile cycling of glycogen in Fibrobacter succinogenes as shown by in situ 1H-NMR and 13C-NMR investigation. Eur. J. Biochem. 207:155-162. - PubMed
-
- Gerngross, T. U., K. D. Snell, O. P. Peoples, A. J. Sinskey, E. Csuhai, S. Masamune, and J. Stubbe. 1994. Overexpression and purification of the soluble polyhydroxyalkanoate synthase from Alcaligenes eutrophus: evidence for a required posttranslational modification for catalytic activity. Biochemistry 33:9311-9320. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

