PhoP-responsive expression of the Salmonella enterica serovar typhimurium slyA gene
- PMID: 12775687
- PMCID: PMC156224
- DOI: 10.1128/JB.185.12.3508-3514.2003
PhoP-responsive expression of the Salmonella enterica serovar typhimurium slyA gene
Abstract
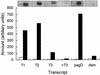
The SlyA protein of Salmonella enterica serovar Typhimurium is a member of the MarR family of transcription regulators and is required for virulence and survival in professional macrophages. Isolated SlyA protein was able to bind a specific DNA target without posttranslational modification. This suggested that SlyA might not be activated by directly sensing an external signal but rather that the intracellular concentration of SlyA is enhanced in appropriate environments through the action of other transcription factors. Analysis of slyA transcription reveals the presence of a promoter region located upstream of the previously recognized SlyA repressed promoter. The newly identified upstream promoter region did not respond to SlyA but was activated by Mg(II) starvation in a PhoP-dependent manner. We present here evidence for a direct link between two transcription factors (PhoP and SlyA) crucial for Salmonella virulence.
Figures

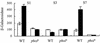




References
-
- Alekshun, M. N., S. B. Levy, T. R. Mealy, B. A. Seaton, and J. F. Head. 2001. The crystal structure of MarR, a regulator of multiple antibiotic resistance, at 2.3 Å resolution. Nat. Struct. Biol. 8:710-714. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

