Toward a detailed computational model for the mammalian circadian clock
- PMID: 12775757
- PMCID: PMC165828
- DOI: 10.1073/pnas.1132112100
Toward a detailed computational model for the mammalian circadian clock
Abstract
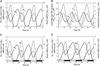
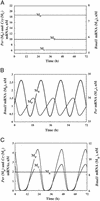
We present a computational model for the mammalian circadian clock based on the intertwined positive and negative regulatory loops involving the Per, Cry, Bmal1, Clock, and Rev-Erb alpha genes. In agreement with experimental observations, the model can give rise to sustained circadian oscillations in continuous darkness, characterized by an antiphase relationship between Per/Cry/Rev-Erbalpha and Bmal1 mRNAs. Sustained oscillations correspond to the rhythms autonomously generated by suprachiasmatic nuclei. For other parameter values, damped oscillations can also be obtained in the model. These oscillations, which transform into sustained oscillations when coupled to a periodic signal, correspond to rhythms produced by peripheral tissues. When incorporating the light-induced expression of the Per gene, the model accounts for entrainment of the oscillations by light-dark cycles. Simulations show that the phase of the oscillations can then vary by several hours with relatively minor changes in parameter values. Such a lability of the phase could account for physiological disorders related to circadian rhythms in humans, such as advanced or delayed sleep phase syndrome, whereas the lack of entrainment by light-dark cycles can be related to the non-24h sleep-wake syndrome. The model uncovers the possible existence of multiple sources of oscillatory behavior. Thus, in conditions where the indirect negative autoregulation of Per and Cry expression is inoperative, the model indicates the possibility that sustained oscillations might still arise from the negative autoregulation of Bmal1 expression.
Figures


References
-
- Young, M. W. & Kay, S. A. (2001) Nat. Rev. Genet. 2, 702-715. - PubMed
-
- Loros, J. J. & Dunlap, J. C. (2001) Annu. Rev. Physiol. 63, 757-794. - PubMed
-
- Mori, T. & Johnson, C. H. (2001) Semin. Cell Dev. Biol. 12, 271-278. - PubMed
-
- Roden, L. C. & Carre, I. A. (2001) Semin. Cell Dev. Biol. 12, 305-315. - PubMed
-
- Reppert, S. & Weaver, D. (2002) Nature 418, 935-941. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

