Interaction between the small-nuclear-RNA cap hypermethylase and the spinal muscular atrophy protein, survival of motor neuron
- PMID: 12776181
- PMCID: PMC1319203
- DOI: 10.1038/sj.embor.embor863
Interaction between the small-nuclear-RNA cap hypermethylase and the spinal muscular atrophy protein, survival of motor neuron
Abstract
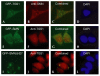
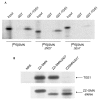
The biogenesis of spliceosomal small nuclear ribonucleoproteins (snRNPs) requires the cytoplasmic assembly of the Sm-core complex, followed by the hypermethylation of the small nuclear RNA (snRNA) 5' cap. Both the Sm-core complex and the snRNA trimethylguanosine cap are required for the efficient nuclear import of snRNPs. Here, we show that trimethylguanosine synthase 1 (TGS1), the human homologue of the yeast snRNA cap hypermethylase, interacts directly with the survival of motor neuron (SMN) protein. Both proteins are similarly distributed, localizing in the cytoplasm and in nuclear Cajal bodies. The interaction between TGS1 and SMN is disrupted by a mutation in SMN that mimics the predominant isoform of the protein that is expressed in patients with the neurodegenerative disease, spinal muscular atrophy. These data indicate that, in addition to its function in cytoplasmic Sm-core assembly, the SMN protein also functions in the recruitment of the snRNA cap hypermethylase.
Figures


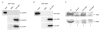

Similar articles
-
The SMN complex is associated with snRNPs throughout their cytoplasmic assembly pathway.Mol Cell Biol. 2002 Sep;22(18):6533-41. doi: 10.1128/MCB.22.18.6533-6541.2002. Mol Cell Biol. 2002. PMID: 12192051 Free PMC article.
-
SMN, the spinal muscular atrophy protein, forms a pre-import snRNP complex with snurportin1 and importin beta.Hum Mol Genet. 2002 Jul 15;11(15):1785-95. doi: 10.1093/hmg/11.15.1785. Hum Mol Genet. 2002. PMID: 12095920 Free PMC article.
-
A 69-kD protein that associates reversibly with the Sm core domain of several spliceosomal snRNP species.J Cell Biol. 1994 Feb;124(3):261-72. doi: 10.1083/jcb.124.3.261. J Cell Biol. 1994. PMID: 8294511 Free PMC article.
-
SMN - A chaperone for nuclear RNP social occasions?RNA Biol. 2017 Jun 3;14(6):701-711. doi: 10.1080/15476286.2016.1236168. Epub 2016 Sep 20. RNA Biol. 2017. PMID: 27648855 Free PMC article. Review.
-
The SMN complex: an assembly machine for RNPs.Cold Spring Harb Symp Quant Biol. 2006;71:313-20. doi: 10.1101/sqb.2006.71.001. Cold Spring Harb Symp Quant Biol. 2006. PMID: 17381311 Review.
Cited by
-
Structural basis for m7G-cap hypermethylation of small nuclear, small nucleolar and telomerase RNA by the dimethyltransferase TGS1.Nucleic Acids Res. 2009 Jul;37(12):3865-77. doi: 10.1093/nar/gkp249. Epub 2009 Apr 22. Nucleic Acids Res. 2009. PMID: 19386620 Free PMC article.
-
Intimate functional interactions between TGS1 and the Smn complex revealed by an analysis of the Drosophila eye development.PLoS Genet. 2020 May 26;16(5):e1008815. doi: 10.1371/journal.pgen.1008815. eCollection 2020 May. PLoS Genet. 2020. PMID: 32453722 Free PMC article.
-
The RNA helicase DDX39 contributes to the nuclear export of spliceosomal U snRNA by loading of PHAX onto RNA.Nucleic Acids Res. 2024 Sep 23;52(17):10668-10682. doi: 10.1093/nar/gkae622. Nucleic Acids Res. 2024. PMID: 39011894 Free PMC article.
-
The right pick: structural basis of snRNA selection by Gemin5.Genes Dev. 2016 Nov 1;30(21):2341-2344. doi: 10.1101/gad.293084.116. Genes Dev. 2016. PMID: 27881598 Free PMC article.
-
Chaperoning ribonucleoprotein biogenesis in health and disease.EMBO Rep. 2007 Apr;8(4):340-5. doi: 10.1038/sj.embor.7400941. EMBO Rep. 2007. PMID: 17401408 Free PMC article. Review.
References
-
- Brahms H., Raymackers J., Union A., de Keyser F., Meheus L. & Lührmann R. (2000) The C-terminal RG dipeptide repeats of the spliceosomal Sm proteins D1 and D3 contain symmetrical dimethylarginines, which form a major B-cell epitope for antism autoantibodies. J. Biol. Chem., 275, 17122–17129. - PubMed
-
- Fischer U. & Lührmann R. (1990) An essential signaling role for the m3G cap in the transport of U1 snRNP to the nucleus. Science, 249, 786–790. - PubMed
-
- Fischer U., Liu Q. & Dreyfuss G. (1997). The SMNsIP1 complex has an essential role in spliceosomal snRNP biogenesis. Cell, 90, 1023–1029. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

