O-mannosylation precedes and potentially controls the N-glycosylation of a yeast cell wall glycoprotein
- PMID: 12776183
- PMCID: PMC1319204
- DOI: 10.1038/sj.embor.embor864
O-mannosylation precedes and potentially controls the N-glycosylation of a yeast cell wall glycoprotein
Abstract
Secretory proteins in yeast are N- and O-glycosylated while they enter the endoplasmic reticulum. N-glycosylation is initiated by the oligosaccharyl transferase complex and O-mannosylation is initiated by distinct O-mannosyltransferase complexes of the protein mannosyl transferase Pmt1/Pmt2 and Pmt4 families. Using covalently linked cell-wall protein 5 (Ccw5) as a model, we show that the Pmt4 and Pmt1/Pmt2 mannosyltransferases glycosylate different domains of the Ccw5 protein, thereby mannosylating several consecutive serine and threonine residues. In addition, it is shown that O-mannosylation by Pmt4 prevents N-glycosylation by blocking the hydroxy amino acid of the single N-glycosylation site present in Ccw5. These data prove that the O- and N-glycosylation machineries compete for Ccw5; therefore O-mannosylation by Pmt4 precedes N-glycosylation.
Figures

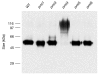
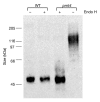


Similar articles
-
Translational Regulation of Pmt1 and Pmt2 by Bfr1 Affects Unfolded Protein O-Mannosylation.Int J Mol Sci. 2019 Dec 10;20(24):6220. doi: 10.3390/ijms20246220. Int J Mol Sci. 2019. PMID: 31835530 Free PMC article.
-
Protein-O-glycosylation in yeast: protein-specific mannosyltransferases.Glycobiology. 1997 Jun;7(4):481-6. doi: 10.1093/glycob/7.4.481. Glycobiology. 1997. PMID: 9184828
-
Monitoring Protein Dynamics in Protein O-Mannosyltransferase Mutants In Vivo by Tandem Fluorescent Protein Timers.Molecules. 2018 Oct 12;23(10):2622. doi: 10.3390/molecules23102622. Molecules. 2018. PMID: 30322079 Free PMC article.
-
Protein O-mannosylation: what we have learned from baker's yeast.Biochim Biophys Acta. 2013 Nov;1833(11):2438-46. doi: 10.1016/j.bbamcr.2013.02.008. Epub 2013 Feb 20. Biochim Biophys Acta. 2013. PMID: 23434682 Review.
-
Protein O-mannosylation: conserved from bacteria to humans.Glycobiology. 2009 Aug;19(8):816-28. doi: 10.1093/glycob/cwp066. Epub 2009 May 9. Glycobiology. 2009. PMID: 19429925 Review.
Cited by
-
Transcription of N- and O-linked mannosyltransferase genes is modulated by the pacC gene in the human dermatophyte Trichophyton rubrum.FEBS Open Bio. 2012 Sep 28;2:294-7. doi: 10.1016/j.fob.2012.09.005. Print 2012. FEBS Open Bio. 2012. PMID: 23772361 Free PMC article.
-
Pathogenesis of Dermatophytosis: Sensing the Host Tissue.Mycopathologia. 2017 Feb;182(1-2):215-227. doi: 10.1007/s11046-016-0057-9. Epub 2016 Sep 2. Mycopathologia. 2017. PMID: 27590362 Review.
-
Architecture and biosynthesis of the Saccharomyces cerevisiae cell wall.Genetics. 2012 Nov;192(3):775-818. doi: 10.1534/genetics.112.144485. Genetics. 2012. PMID: 23135325 Free PMC article. Review.
-
Endoplasmic reticulum glucosidases and protein quality control factors cooperate to establish biotrophy in Ustilago maydis.Plant Cell. 2013 Nov;25(11):4676-90. doi: 10.1105/tpc.113.115691. Epub 2013 Nov 26. Plant Cell. 2013. PMID: 24280385 Free PMC article.
-
Membrane association is a determinant for substrate recognition by PMT4 protein O-mannosyltransferases.Proc Natl Acad Sci U S A. 2007 May 8;104(19):7827-32. doi: 10.1073/pnas.0700374104. Epub 2007 Apr 30. Proc Natl Acad Sci U S A. 2007. PMID: 17470820 Free PMC article.
References
-
- Brockhausen I. (1995) in Glycoproteins, Vol. 29a (eds Montreuil, J., Vliegenthart, J.F.G. & Schachter, H.), 201–259. Elsevier, Amsterdam, The Netherlands.
-
- Chiba A., Matsumura K., Yamada H., Inazu T., Shimizu T., Kusunoki S., Kanazawa I., Kobata A. & Endo T. (1997) Structures of sialylated O-linked oligosaccharides of bovine peripheral nerve α-dystroglycan: the role of a novel O-mannosyl-type oligosaccharide in the binding of α-dystroglycan with laminin. J. Biol. Chem., 272, 2156–2162. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

