Human arteries engineered in vitro
- PMID: 12776184
- PMCID: PMC1319197
- DOI: 10.1038/sj.embor.embor847
Human arteries engineered in vitro
Abstract
There is a pressing need to develop methods to engineer small-calibre arteries for bypass surgery. We hypothesized that the rate-limiting step that has thwarted previous attempts to engineer such vessels from non-neonatal tissues is the limited proliferative capacity of smooth muscle cells (SMCs), which are the main cellular component of these vessels. Ectopic expression of the human telomerase reverse transcriptase subunit (hTERT) has been shown recently to extend the lifespan of certain human cells. We therefore introduced hTERT into human SMCs and found that the resulting cells proliferated far beyond their normal lifespan but retained characteristics of normal control SMCs. Importantly, using these non-neonatal SMCs, we were able to engineer mechanically robust human vessels, a crucial step towards creating arteries of clinical value for bypass surgery.
Figures

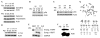

References
-
- Bierman E.L. (1978) The effect of donor age on the in vitro life span of cultured human arterial smooth-muscle cells. In Vitro, 14, 951–955. - PubMed
-
- Bodnar A.G., Ouellette M., Frolkis M., Holt S.E., Chiu C.P., Morin G.B., Harley C.B., Shay J.W., Lichtsteiner S. & Wright W.E. (1998) Extension of lifespan by introduction of telomerase into normal human cells. Science, 279, 349–352. - PubMed
-
- Bonin L.R., Madden K., Shera K., Ihle J., Matthews C., Aziz S., Perez-Reyes N., McDougall J.K. & Conroy S.C. (1999) Generation and characterization of human smooth muscle cell lines derived from atherosclerotic plaque. Arterioscler. Thromb. Vasc. Biol, 19, 575–587. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

