Overexpression of Krüppel-like factor 4 in the human colon cancer cell line RKO leads to reduced tumorigenecity
- PMID: 12776194
- PMCID: PMC2275074
- DOI: 10.1038/sj.onc.1206413
Overexpression of Krüppel-like factor 4 in the human colon cancer cell line RKO leads to reduced tumorigenecity
Abstract
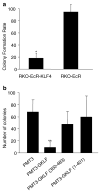
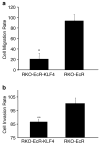
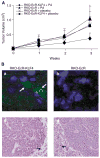
Krüppel-like factor 4 (KLF4) is a zinc-finger-containing transcription factor, the expression of which is enriched in the postmitotic cells of the intestinal epithelium. KLF4 is a target gene of the tumor suppressor adenomatous polyposis coli (APC). We sought to determine the role of KLF4 in suppressing the tumorigenecity of RKO colon cancer cells, which do not express KLF4. We utilized an established system in RKO cells, in which an inducible promoter controls expression of KLF4. Four independent assays were used to assess the effects of KLF4 induction on tumor cells. We find that KLF4 overexpression reduces colony formation, cell migration and invasion, and in vivo tumorigenecity. The mechanism of action of KLF4 does not involve apoptosis. These findings, along with our previous findings that KLF4 induces G1/S arrest, suggest that KLF4 is a cell cycle checkpoint protein that can reduce tumorigenecity of colon cancer cells.
Figures
Similar articles
-
Expression of the gut-enriched Krüppel-like factor (Krüppel-like factor 4) gene in the human colon cancer cell line RKO is dependent on CDX2.Oncogene. 2001 Aug 9;20(35):4884-90. doi: 10.1038/sj.onc.1204645. Oncogene. 2001. PMID: 11521200 Free PMC article.
-
Krüppel-like factor 4 is transactivated by butyrate in colon cancer cells.J Nutr. 2004 Apr;134(4):792-8. doi: 10.1093/jn/134.4.792. J Nutr. 2004. PMID: 15051827
-
Krüppel-like factor 4 (gut-enriched Krüppel-like factor) inhibits cell proliferation by blocking G1/S progression of the cell cycle.J Biol Chem. 2001 Aug 10;276(32):30423-8. doi: 10.1074/jbc.M101194200. Epub 2001 Jun 4. J Biol Chem. 2001. PMID: 11390382 Free PMC article.
-
Notch inhibits expression of the Krüppel-like factor 4 tumor suppressor in the intestinal epithelium.Mol Cancer Res. 2008 Dec;6(12):1920-7. doi: 10.1158/1541-7786.MCR-08-0224. Mol Cancer Res. 2008. PMID: 19074836 Free PMC article.
-
Roles of Krüpel-like factor 4 in normal homeostasis, cancer and stem cells.Acta Biochim Biophys Sin (Shanghai). 2008 Jul;40(7):554-64. doi: 10.1111/j.1745-7270.2008.00439.x. Acta Biochim Biophys Sin (Shanghai). 2008. PMID: 18604447 Free PMC article. Review.
Cited by
-
Krüppel-like factor 4 acts as an oncogene in colon cancer stem cell-enriched spheroid cells.PLoS One. 2013;8(2):e56082. doi: 10.1371/journal.pone.0056082. Epub 2013 Feb 13. PLoS One. 2013. PMID: 23418515 Free PMC article.
-
Functional effects of GRM1 suppression in human melanoma cells.Mol Cancer Res. 2012 Nov;10(11):1440-50. doi: 10.1158/1541-7786.MCR-12-0158. Epub 2012 Jul 13. Mol Cancer Res. 2012. PMID: 22798429 Free PMC article.
-
miR-7 suppresses brain metastasis of breast cancer stem-like cells by modulating KLF4.Cancer Res. 2013 Feb 15;73(4):1434-44. doi: 10.1158/0008-5472.CAN-12-2037. Epub 2013 Feb 5. Cancer Res. 2013. PMID: 23384942 Free PMC article.
-
MicroRNA-10b promotes migration and invasion through KLF4 in human esophageal cancer cell lines.J Biol Chem. 2010 Mar 12;285(11):7986-94. doi: 10.1074/jbc.M109.062877. Epub 2010 Jan 14. J Biol Chem. 2010. PMID: 20075075 Free PMC article.
-
Human Xylosyltransferase I-An Important Linker between Acute Senescence and Fibrogenesis.Biomedicines. 2023 Feb 4;11(2):460. doi: 10.3390/biomedicines11020460. Biomedicines. 2023. PMID: 36830996 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

