The future of bacteriophage biology
- PMID: 12776216
- PMCID: PMC7097159
- DOI: 10.1038/nrg1089
The future of bacteriophage biology
Abstract
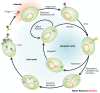
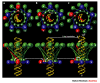
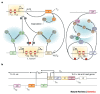
After an illustrious history as one of the primary tools that established the foundations of molecular biology, bacteriophage research is now undergoing a renaissance in which the primary focus is on the phages themselves rather than the molecular mechanisms that they explain. Studies of the evolution of phages and their role in natural ecosystems are flourishing. Practical questions, such as how to use phages to combat human diseases that are caused by bacteria, how to eradicate phage pests in the food industry and what role they have in the causation of human diseases, are receiving increased attention. Phages are also useful in the deeper exploration of basic molecular and biophysical questions.
Figures
References
-
- Twort FW. An investigation on the nature of ultramicroscopic viruses. Lancet. 1916;189:1241–1243.
-
- Campbell A, Botstein D. Lambda II. 1983. pp. 356–381.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

