A crucial role for profilin-actin in the intracellular motility of Listeria monocytogenes
- PMID: 12776739
- PMCID: PMC1319178
- DOI: 10.1038/sj.embor.embor823
A crucial role for profilin-actin in the intracellular motility of Listeria monocytogenes
Abstract
We have examined the effect of covalently crosslinked profilin-actin (PxA), which closely matches the biochemical properties of ordinary profilin-actin and interferes with actin polymerization in vitro and in vivo, on Listeria monocytogenes motility. PxA caused a marked reduction in bacterial motility, which was accompanied by the detachment of bacterial tails. The effect of PxA was dependent on its binding to proline-rich sequences, as shown by the inability of PH133SxA, which cannot interact with such sequences, to impair Listeria motility. PxA did not alter the motility of a Listeria mutant that is unable to recruit Ena (Enabled)/VASP (vasodilator-stimulated phosphoprotein) proteins and profilin to its surface. Finally, PxA did not block the initiation of actin-tail formation, indicating that profilin-actin is only required for the elongation of actin filaments at the bacterial surface. Our findings provide further evidence that profilin-actin is important for actin-based processes, and show that it has a key function in Listeria motility.
Figures

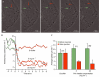

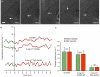

Similar articles
-
Profilin interacts with the Gly-Pro-Pro-Pro-Pro-Pro sequences of vasodilator-stimulated phosphoprotein (VASP): implications for actin-based Listeria motility.Biochemistry. 1997 Jul 8;36(27):8384-92. doi: 10.1021/bi970065n. Biochemistry. 1997. PMID: 9204886
-
Role of proteins of the Ena/VASP family in actin-based motility of Listeria monocytogenes.J Cell Biol. 1999 Mar 22;144(6):1245-58. doi: 10.1083/jcb.144.6.1245. J Cell Biol. 1999. PMID: 10087267 Free PMC article.
-
Listeria monocytogenes intracellular migration: inhibition by profilin, vitamin D-binding protein and DNase I.Cell Motil Cytoskeleton. 1995;30(1):38-49. doi: 10.1002/cm.970300106. Cell Motil Cytoskeleton. 1995. PMID: 7728867
-
Intracellular motility. Profilin puts pathogens on the actin drive.Curr Biol. 1994 May 1;4(5):465-8. doi: 10.1016/s0960-9822(00)00105-6. Curr Biol. 1994. PMID: 7922367 Review.
-
How the Listeria monocytogenes ActA protein converts actin polymerization into a motile force.Trends Microbiol. 1997 Jul;5(7):272-6. doi: 10.1016/S0966-842X(97)01048-2. Trends Microbiol. 1997. PMID: 9234509 Review.
Cited by
-
Functional Characterization of an Arabidopsis Profilin Protein as a Molecular Chaperone under Heat Shock Stress.Molecules. 2022 Sep 6;27(18):5771. doi: 10.3390/molecules27185771. Molecules. 2022. PMID: 36144503 Free PMC article.
-
PFN2 and NAA80 cooperate to efficiently acetylate the N-terminus of actin.J Biol Chem. 2020 Dec 4;295(49):16713-16731. doi: 10.1074/jbc.RA120.015468. Epub 2020 Sep 25. J Biol Chem. 2020. PMID: 32978259 Free PMC article.
-
Integration of linear and dendritic actin nucleation in Nck-induced actin comets.Mol Biol Cell. 2016 Jan 15;27(2):247-59. doi: 10.1091/mbc.E14-11-1555. Epub 2015 Nov 25. Mol Biol Cell. 2016. PMID: 26609071 Free PMC article.
-
Defining a core set of actin cytoskeletal proteins critical for actin-based motility of Rickettsia.Cell Host Microbe. 2010 May 20;7(5):388-98. doi: 10.1016/j.chom.2010.04.008. Cell Host Microbe. 2010. PMID: 20478540 Free PMC article.
-
Loss of cytoskeletal transport during egress critically attenuates ectromelia virus infection in vivo.J Virol. 2012 Jul;86(13):7427-43. doi: 10.1128/JVI.06636-11. Epub 2012 Apr 24. J Virol. 2012. PMID: 22532690 Free PMC article.
References
-
- Björkegren-sjögren C., Korenbaum E., Nordberg P., Lindberg U. & Karlsson R. (1997) Isolation and characterization of two mutants of human profilin I that do not bind poly(L-proline). FEBS Lett., 418, 258–264. - PubMed
-
- Carlsson L., Nystrom L.E., Sundkvist I., Markey F. & Lindberg U. (1977) Actin polymerizability is influenced by profilin, a low molecular weight protein in non-muscle cells. J. Mol. Biol., 115, 465–483. - PubMed
-
- Cedergren-Zeppezauer E.S., Goonesekere N.C., Rozycki M.D., Myslik J.C., Dauter Z., Lindberg U. & Schutt C.E. (1994) Crystallization and structure determination of bovine profilin at 2.0 Å resolution. J. Mol. Biol., 240, 459–475. - PubMed
-
- Choidas A., Jungbluth A., Sechi A., Murphy J., Ullrich A. & Marriott G. (1998) The suitability and application of a GFP–actin fusion protein for long-term imaging of the organization and dynamics of the cytoskeleton in mammalian cells. Eur. J. Cell Biol., 77, 81–90. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

