Discovery of diverse thyroid hormone receptor antagonists by high-throughput docking
- PMID: 12777627
- PMCID: PMC165879
- DOI: 10.1073/pnas.1131854100
Discovery of diverse thyroid hormone receptor antagonists by high-throughput docking
Abstract
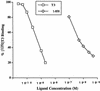
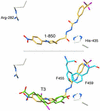
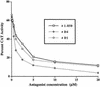
Treatment of hyperthyroidism, a common clinical condition that can have serious manifestations in the elderly, has remained essentially unchanged for >30 years. Directly antagonizing the effect of the thyroid hormone at the receptor level may be a significant improvement for the treatment of hyperthyroid patients. We built a computer model of the thyroid hormone receptor (TR) ligand-binding domain in its predicted antagonist-bound conformation and used a virtual screening algorithm to select 100 TR antagonist candidates out of a library of >250,000 compounds. We were able to obtain 75 of the compounds selected in silico and studied their ability to act as antagonists by using cultured cells that express TR. Fourteen of these compounds were found to antagonize the effect of T3 on TR with IC50s ranging from 1.5 to 30 microM. A small virtual library of compounds, derived from the highest affinity antagonist (1-850) that could be rapidly synthesized, was generated. A second round of virtual screening identified new compounds with predicted increased antagonist activity. These second generation compounds were synthesized, and their ability to act as TR antagonists was confirmed by transfection and receptor binding experiments. The extreme structural diversity of the antagonist compounds shows how receptor-based virtual screening can identify diverse chemistries that comply with the structural rules of TR antagonism.
Figures
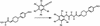
Comment in
-
Making virtual screening a reality.Proc Natl Acad Sci U S A. 2003 Jun 10;100(12):6902-3. doi: 10.1073/pnas.1332743100. Epub 2003 Jun 2. Proc Natl Acad Sci U S A. 2003. PMID: 12782796 Free PMC article. No abstract available.
Similar articles
-
In silico modelling of prostacyclin and other lipid mediators to nuclear receptors reveal novel thyroid hormone receptor antagonist properties.Prostaglandins Other Lipid Mediat. 2016 Jan;122:18-27. doi: 10.1016/j.prostaglandins.2015.12.002. Epub 2015 Dec 11. Prostaglandins Other Lipid Mediat. 2016. PMID: 26686607
-
Design of thyroid hormone receptor antagonists from first principles.J Steroid Biochem Mol Biol. 2002 Dec;83(1-5):59-73. doi: 10.1016/s0960-0760(02)00270-4. J Steroid Biochem Mol Biol. 2002. PMID: 12650702 Review.
-
Structure-based design and synthesis of a thyroid hormone receptor (TR) antagonist.Endocrinology. 2002 Feb;143(2):517-24. doi: 10.1210/endo.143.2.8617. Endocrinology. 2002. PMID: 11796506
-
Discovery of small molecule inhibitors of the interaction of the thyroid hormone receptor with transcriptional coregulators.J Biol Chem. 2005 Dec 30;280(52):43048-55. doi: 10.1074/jbc.M506693200. Epub 2005 Oct 31. J Biol Chem. 2005. PMID: 16263725
-
Thyroid hormone antagonists: potential medical applications and structure activity relationships.Curr Med Chem. 2009;16(25):3258-66. doi: 10.2174/092986709788803277. Epub 2009 Sep 1. Curr Med Chem. 2009. PMID: 19548868 Review.
Cited by
-
Rapid signaling at the plasma membrane by a nuclear receptor for thyroid hormone.Proc Natl Acad Sci U S A. 2006 Mar 28;103(13):5197-201. doi: 10.1073/pnas.0600089103. Epub 2006 Mar 20. Proc Natl Acad Sci U S A. 2006. PMID: 16549781 Free PMC article.
-
Dioxin Exposure Alters Molecular and Morphological Responses to Thyroid Hormone in Xenopus laevis Cultured Cells and Prometamorphic Tadpoles.Toxicol Sci. 2018 Jan 1;161(1):196-206. doi: 10.1093/toxsci/kfx213. Toxicol Sci. 2018. PMID: 29294139 Free PMC article.
-
Understanding nuclear receptors using computational methods.Drug Discov Today. 2009 May;14(9-10):486-94. doi: 10.1016/j.drudis.2009.03.003. Epub 2009 Mar 11. Drug Discov Today. 2009. PMID: 19429508 Free PMC article. Review.
-
Compound activity prediction using models of binding pockets or ligand properties in 3D.Curr Top Med Chem. 2012;12(17):1869-82. doi: 10.2174/156802612804547335. Curr Top Med Chem. 2012. PMID: 23116466 Free PMC article. Review.
-
Data-Driven Strategies for Accelerated Materials Design.Acc Chem Res. 2021 Feb 16;54(4):849-860. doi: 10.1021/acs.accounts.0c00785. Epub 2021 Feb 2. Acc Chem Res. 2021. PMID: 33528245 Free PMC article.
References
-
- DeGroot, L. J. (2001) Endocrinology (Saunders, Philadelphia), 4th Ed.
-
- Braverman, L. E., Utiger, R. D., Ingbar, S. H. & Werner, S. C., eds. (2000) Werner and Ingbar's the Thyroid: A Fundamental and Clinical Text (Lippincott Williams & Wilkins, Baltimore), 8th Ed.
-
- Wilson J. D., ed. (1998) Textbook of Endocrinology (Saunders, Philadelphia).
-
- Yen, P. M. (2001) Physiol. Rev. 81, 1097-1142. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

