Physiological and anatomical link between Parkinson-like disease and REM sleep behavior disorder
- PMID: 12777684
- PMCID: PMC8801047
- DOI: 10.1385/MN:27:2:137
Physiological and anatomical link between Parkinson-like disease and REM sleep behavior disorder
Abstract
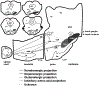
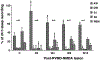
Parkinson's disease (PD) is a progressive neurodegenerative disease that is caused by a loss of neurons in the ventral midbrain. Parkinsonian patients often experience insomnia, parasomnias, and daytime somnolence. REM sleep behavior disorder (RBD) is characterized by vigorous movements during REM sleep, and may also be caused by neuronal degeneration in the central nervous system (CNS); however, the site of degeneration remains unclear. Both Parkinsonism and RBD become more prevalent with aging, with onset usually occurring in the sixties. Recent findings show that many individuals with RBD eventually develop Parkinsonism. Conversely, it is also true that certain patients diagnosed with Parkinsonism subsequently develop RBD. Postmortem examination reveals that Lewy bodies, Lewy neurites, and alpha-synuclein are found in brainstem nuclei in both Parkinsonism and RBD patients. In this article, we will discuss evidence that Parkinsonism and RBD are physiologically and anatomically linked, based on our animal experiments and other studies on human patients.
Figures
References
-
- German DC, Dubach M, Askari S, Apeciale SG, and Bowden DM (1988) 1-methyl-4phenyl-1,2,3,6-tetrahydropyridine-induced Parkinsonian syndrome in Macaca fascicularis: which midbrain dopaminergic neurons are lost? Neuroscience 24, 161–174. - PubMed
-
- Janowsky A, Vocci F, Berger P, Angel I, Zelnik N, Kleinman JE, et al. (1987) [3H]GBR-12935 binding to the dopamine transporter is decreased in the caudate nucleus in Parkinson’s disease. J. Neurochem 49, 617–621. - PubMed
-
- Lee CS, Samii A, Sossi V, Ruth TJ, Schulzer M, Holden JE, et al. (2000) In vivo positron emission tomographic evidence for compensatory changes in presynaptic dopaminergic nerve terminals in Parkinson’s disease. Ann. Neurol 47, 493–503. - PubMed
-
- Varrone A, Marek KL, Jennings D, Innis RB, and Seibyl JP (2001) [(123)I]beta-CIT SPECT imaging demonstrates reduced density of striatal dopamine transporters in Parkinson’s disease and multiple system atrophy. Mov. Disord 16, 1023–1032. - PubMed
-
- Pearce RKB, Seeman P, Jellinger K, and Tourtellotte WW (1990) Dopamine uptake sites and dopamine receptors in Parkinson’s disease and schizophrenia. Eur. Neurol 30, Suppl 1, 9–14. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

