The coxsackievirus and adenovirus receptor acts as a tumour suppressor in malignant glioma cells
- PMID: 12778071
- PMCID: PMC2741053
- DOI: 10.1038/sj.bjc.6600932
The coxsackievirus and adenovirus receptor acts as a tumour suppressor in malignant glioma cells
Abstract
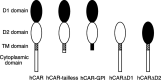
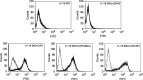
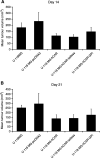
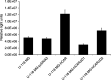
The coxsackievirus and adenovirus receptor (CAR) is a membrane glycoprotein with a cytoplasmic domain, a transmembrane domain and an extracellular region consisting of two immunoglobulin-like domains, an amino-terminal immunoglobulin variable (IgV)-related domain (D1), which is distal to the cell surface, and a proximal IgC2 domain (D2). The coxsackievirus and adenovirus receptor has been shown to exhibit tumour suppression activity in human bladder and prostate cancer cells. In the current paper, we demonstrate that CAR is a tumour suppressor in glioma cells and that the extracellular D2 domain is not required for this inhibitory effect. This finding provides a biological basis for the observation that expression of CAR is downregulated in malignant glioma cells. This suggests that strategies to redirect adenoviruses to achieve CAR-independent infection will be necessary to realise the full potential of adenoviral vectors for cancer gene therapy.
Figures
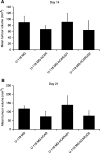
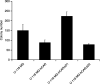
References
-
- Anon (1998) Use and application of adenovirus expression vectors. In Cells: A Laboratory Manual, Leinwand LA (ed) Vol. 2, pp 90.1–90.28. New York: Cold Spring Harbor Laboratory Press
-
- Barnett BG, Crews CJ, Douglas JT (2002) Targeted adenoviral vectors. Biochim Biophys Acta 1575: 1–14 - PubMed
-
- Blackwell JL, Miller CR, Douglas JT, Li H, Reynolds PN, Carroll WR, Peters GE, Strong TV, Curiel DT (1999) Retargeting to EGFR enhances adenovirus infection of squamous cell carcinoma of the head and neck. Arch Otolaryngol Head Neck Surg 125: 856–863 - PubMed
-
- Dethlefsen LA, Prewitt JM, Mendelsohn ML (1968) Analysis of tumor growth curves. J Natl Cancer Inst 40: 389–405 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

