The production of tumour necrosis factor-alpha is decreased in peripheral blood mononuclear cells from multidrug-resistant tuberculosis patients following stimulation with the 30-kDa antigen of Mycobacterium tuberculosis
- PMID: 12780691
- PMCID: PMC1808731
- DOI: 10.1046/j.1365-2249.2003.02172.x
The production of tumour necrosis factor-alpha is decreased in peripheral blood mononuclear cells from multidrug-resistant tuberculosis patients following stimulation with the 30-kDa antigen of Mycobacterium tuberculosis
Abstract
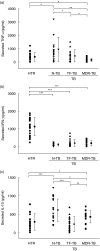
The clearance of intracellular bacteria requires the appropriate induction of proinflammatory cytokines and chemokines to recruit macrophages and T cells to the site of infection. In this study, we investigated the production of tumour necrosis factor (TNF)-alpha, interleukin (IL)-8 and interferon (IFN)-gamma by the peripheral blood mononuclear cells (PBMC) of patients with multidrug-resistant tuberculosis (MDR-TB) in response to in vitro stimulation with the 30-kDa antigen of Mycobacterium tuberculosis. The results were compared with those from cases of newly diagnosed TB (N-TB) and TB with treatment failure (TF-TB), and healthy tuberculin reactors (HTR). The most significantly depressed TNF-alpha levels were found in MDR-TB patients. IFN-gamma production was depressed significantly in all groups of TB patients compared with the HTR group. TNF-alpha secretion in response to the 30-kDa antigen was unchanged by coculturing with recombinant human interferon (rhIFN)-gamma, and was increased dramatically following IL-10 neutralization with an anti-human IL-10 antibody. The IL-8 levels were depressed significantly in MDR-TB patients compared with N-TB patients, but were similar to the IL-8 levels in TF-TB patients. Furthermore, rhTNF-alpha directly increased IL-8 secretion, and neutralizing antibody to TNF-alpha inhibited IL-8 production by the PBMC of MDR-TB patients that were stimulated with the 30-kDa antigen. Taken together, these data suggest that the PBMC of MDR-TB patients typically show TNF-alpha depression in response to the 30-kDa antigen, and this effect is modulated by IL-10. In addition, we highlight the role of TNF-alpha in IL-8 secretion in MDR-TB patients.
Figures
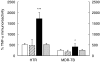
 , 30-kDa + rhIFN-γ; ▪, 30-kDa + α-IL-10;
, 30-kDa + rhIFN-γ; ▪, 30-kDa + α-IL-10;  , 30-kDa + control IgG.
, 30-kDa + control IgG.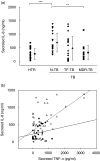

References
-
- Raviglione MC, Snider DE, Jr, Kochi A. Global epidemiology of tuberculosis. Morbidity and mortality of a worldwide epidemic. JAMA. 1995;273:220–6. - PubMed
-
- McDyer JF, Hackley MN, Walsh TE, Cook JL, Seder RA. Patients with multidrug-resistant tuberculosis with low CD4+ T cell counts have impaired Th1 responses. J Immunol. 1997;158:492–500. - PubMed
-
- Keane J, Gershon S, Wise RP, et al. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N Engl J Med. 2001;345:1098–104. - PubMed
-
- Flynn JL, Goldstein MM, Chan J, et al. Tumor necrosis factor-α is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity. 1995;2:561–72. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

