Specific lytic activity against mycobacterial antigens is inversely correlated with the severity of tuberculosis
- PMID: 12780692
- PMCID: PMC1808720
- DOI: 10.1046/j.1365-2249.2003.02176.x
Specific lytic activity against mycobacterial antigens is inversely correlated with the severity of tuberculosis
Abstract
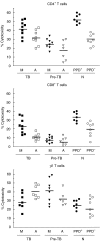
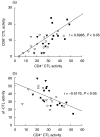
The ability of peripheral blood mononuclear cells (PBMC) from patients with active tuberculosis to display cytotoxic responses against autologous Mycobacterium tuberculosis (Mtb)-pulsed macrophages was evaluated. Non-MHC restricted cell-dependent lytic activity was observed in ex vivo effector cells from tuberculosis patients and was mediated mainly by CD3(+)gammadelta TCR(+) T (gammadelta T) cells bearing CD56 and/or CD16 molecules. MHC-restricted and non-MHC restricted cytotoxic T cells (CTL) were differentially expanded upon stimulation with Mtb in tuberculosis patients and normal controls (N). Class-I restricted CD8(+) CTL and class-II restricted CD4(+) CTL were generated in PPD(+)N and to a lesser extent in PPD(-)N. Mtb-stimulated effector cells from tuberculosis patients became progressively non-MHC restricted CD4(-)CD8(-)gammadelta T cells, while lytic activity of CD4(+) and CD8(+)CTL decreased gradually as the disease became more severe. On the other hand, target cells were lysed by ex vivo cells from tuberculosis patients through the Fas-FasL and perforin pathways. Mtb-induced CD4(+) CTL from tuberculosis patients and N controls preferentially employed the Fas-FasL mechanism. Mtb-induced CD8(+) CTL effector cells from patients used the perforin-based mechanism while cells from N controls also used the Fas-FasL pathway. While Mtb-induced gammadelta CTL from patients and PPD(-)N employed the latter mechanism cells from PPD(+)N individuals also used the perforin pathway. It can be concluded that shifts in the CTL response and the cytolytic mechanisms take place as the pulmonary involvement becomes more severe.
Figures

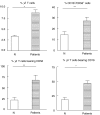

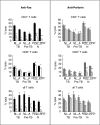
 ). Results are expressed as percentage of lysis (× ± s.e.m.). Statistical differences between percentage lysis from (E + anti-Fas treated antigen-pulsed target cells) or from (E + antigen-pulsed target cells + antiperforin) and percentage lysis from (E + antigen-pulsed target cells): *P < 0·05, **P < 0·01.
). Results are expressed as percentage of lysis (× ± s.e.m.). Statistical differences between percentage lysis from (E + anti-Fas treated antigen-pulsed target cells) or from (E + antigen-pulsed target cells + antiperforin) and percentage lysis from (E + antigen-pulsed target cells): *P < 0·05, **P < 0·01.Similar articles
-
IL-10 down-regulates costimulatory molecules on Mycobacterium tuberculosis-pulsed macrophages and impairs the lytic activity of CD4 and CD8 CTL in tuberculosis patients.Clin Exp Immunol. 2004 Oct;138(1):128-38. doi: 10.1111/j.1365-2249.2004.02577.x. Clin Exp Immunol. 2004. PMID: 15373915 Free PMC article.
-
Human alveolar T lymphocyte responses to Mycobacterium tuberculosis antigens: role for CD4+ and CD8+ cytotoxic T cells and relative resistance of alveolar macrophages to lysis.J Immunol. 1997 Jul 1;159(1):290-7. J Immunol. 1997. PMID: 9200465
-
CD4(+) and CD8(+) T cells kill intracellular Mycobacterium tuberculosis by a perforin and Fas/Fas ligand-independent mechanism.J Immunol. 2001 Sep 1;167(5):2734-42. doi: 10.4049/jimmunol.167.5.2734. J Immunol. 2001. PMID: 11509617
-
Mechanism and biological significance of CD4-mediated cytotoxicity.Immunol Rev. 1995 Aug;146:57-79. doi: 10.1111/j.1600-065x.1995.tb00684.x. Immunol Rev. 1995. PMID: 7493761 Review.
-
Cytotoxic T lymphocytes in resistance to tuberculosis.Adv Exp Med Biol. 1998;452:85-101. doi: 10.1007/978-1-4615-5355-7_11. Adv Exp Med Biol. 1998. PMID: 9889963 Review.
Cited by
-
Rhodococcus equi-specific cytotoxic T lymphocytes in immune horses and development in asymptomatic foals.Infect Immun. 2005 Apr;73(4):2083-93. doi: 10.1128/IAI.73.4.2083-2093.2005. Infect Immun. 2005. PMID: 15784549 Free PMC article.
-
Impaired expression of perforin and granulysin in CD8+ T cells at the site of infection in human chronic pulmonary tuberculosis.Infect Immun. 2007 Nov;75(11):5210-22. doi: 10.1128/IAI.00624-07. Epub 2007 Jul 30. Infect Immun. 2007. PMID: 17664265 Free PMC article.
-
Diagnosis of gastrointestinal tuberculosis: Using cytomorphological, microbiological, immunological and molecular techniques - A study from Central India.Indian J Clin Biochem. 2010 Apr;25(2):158-63. doi: 10.1007/s12291-010-0029-7. Epub 2010 May 27. Indian J Clin Biochem. 2010. PMID: 23105903 Free PMC article.
-
CTLs: Killers of intracellular bacteria.Front Cell Infect Microbiol. 2022 Oct 26;12:967679. doi: 10.3389/fcimb.2022.967679. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36389159 Free PMC article. Review.
-
Nonclassical T cells and their antigens in tuberculosis.Cold Spring Harb Perspect Med. 2014 Jul 24;4(9):a018473. doi: 10.1101/cshperspect.a018473. Cold Spring Harb Perspect Med. 2014. PMID: 25059739 Free PMC article. Review.
References
-
- Flynn J, Chan J. Immunology of tuberculosis. Ann Rev Immunol. 2001;19:93–129. - PubMed
-
- Pithie AD, Lammas DA, Fazel N, et al. CD4+ cytolytic T cells can destroy autologous and MHC-matched macrophages but fail to kill intracellular Mycobacterium bovis-BCG. FEMS Immunol Med Microbiol. 1995;11:145–54. - PubMed
-
- Orme I, Miller E, Roberts A, et al. T lymphocytes mediating protection and cellular cytolysis during the course of Mycobacterium tuberculosis infection. Evidence for different kinetics and recognition of a wide spectrum of protein antigens. J Immunol. 1992;148:189–96. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

