Polymorphisms in Chlamydia trachomatis tryptophan synthase genes differentiate between genital and ocular isolates
- PMID: 12782678
- PMCID: PMC156111
- DOI: 10.1172/JCI17993
Polymorphisms in Chlamydia trachomatis tryptophan synthase genes differentiate between genital and ocular isolates
Abstract
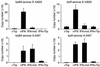
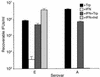
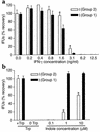
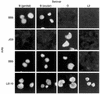
We previously reported that laboratory reference strains of Chlamydia trachomatis differing in infection organotropism correlated with inactivating mutations in the pathogen's tryptophan synthase (trpBA) genes. Here, we have applied functional genomics to extend this work and find that the paradigm established for reference serovars also applies to clinical isolates - specifically, all ocular trachoma isolates tested have inactivating mutations in the synthase, whereas all genital isolates encode a functional enzyme. Moreover, functional enzyme activity was directly correlated to IFN-gamma resistance through an indole rescue mechanism. Hence, a strong selective pressure exists for genital strains to maintain a functional synthase capable of using indole for tryptophan biosynthesis. The fact that ocular serovars (serovar B) isolated from the genital tract were found to possess a functional synthase provided further persuasive evidence of this association. These results argue that there is an important host-parasite relationship between chlamydial genital strains and the human host that determines organotropism of infection and the pathophysiology of disease. We speculate that this relationship involves the production of indole by components of the vaginal microbial flora, allowing chlamydiae to escape IFN-gamma-mediated eradication and thus establish persistent infection.
Figures




Comment in
-
New insights into a persistent problem -- chlamydial infections.J Clin Invest. 2003 Jun;111(11):1647-9. doi: 10.1172/JCI18770. J Clin Invest. 2003. PMID: 12782667 Free PMC article. Review.
References
-
- Schachter, J. 1999. Infection and disease epidemiology. American Society for Microbiology Press. Washington, DC, USA. 139–170.
-
- Stephens, R.S. 1999. Genomic autobiographies of Chlamydiae. American Society for Microbiology Press. Washington, DC, USA. 9–27.
-
- An BB, Adamis AP. Chlamydial ocular diseases. Int. Ophthalmol. Clin. 1998;38:221–230. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

