The alpha(1A/C)- and alpha(1B)-adrenergic receptors are required for physiological cardiac hypertrophy in the double-knockout mouse
- PMID: 12782680
- PMCID: PMC156101
- DOI: 10.1172/JCI16100
The alpha(1A/C)- and alpha(1B)-adrenergic receptors are required for physiological cardiac hypertrophy in the double-knockout mouse
Abstract
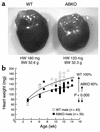
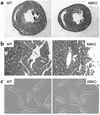
Catecholamines and alpha(1)-adrenergic receptors (alpha(1)-ARs) cause cardiac hypertrophy in cultured myocytes and transgenic mice, but heart size is normal in single KOs of the main alpha(1)-AR subtypes, alpha(1A/C) and alpha(1B). Here we tested whether alpha(1)-ARs are required for developmental cardiac hypertrophy by generating alpha(1A/C) and alpha(1B) double KO (ABKO) mice, which had no cardiac alpha(1)-AR binding. In male ABKO mice, heart growth after weaning was 40% less than in WT, and the smaller heart was due to smaller myocytes. Body and other organ weights were unchanged, indicating a specific effect on the heart. Blood pressure in ABKO mice was the same as in WT, showing that the smaller heart was not due to decreased load. Contractile function was normal by echocardiography in awake mice, but the smaller heart and a slower heart rate reduced cardiac output. alpha(1)-AR stimulation did not activate extracellular signal-regulated kinase (Erk) and downstream kinases in ABKO myocytes, and basal Erk activity was lower in the intact ABKO heart. In female ABKO mice, heart size was normal, even after ovariectomy. Male ABKO mice had reduced exercise capacity and increased mortality with pressure overload. Thus, alpha(1)-ARs in male mice are required for the physiological hypertrophy of normal postnatal cardiac development and for an adaptive response to cardiac stress.
Figures




References
-
- Rakusan, K. 1984. Cardiac growth, maturation and aging. In Growth of the heart in health and disease. R. Zak, editor. Raven Press. New York, New York, USA. 131–164.
-
- Simpson PC, Kariya K, Karns LR, Long CS. The α1-adrenergic receptor in left ventricular hypertrophy. Journal of Vascular Medicine and Biology. 1990;2:236–246.
-
- Rapacciuolo A, et al. Important role of endogenous norepinephrine and epinephrine in the development of in vivo pressure-overload cardiac hypertrophy. J. Am. Coll. Cardiol. 2001;38:876–882. - PubMed
-
- Thomas SA, Matsumoto AM, Palmiter RD. Noradrenaline is essential for mouse fetal development. Nature. 1995;374:643–646. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

