Vaccine immunity to pathogenic fungi overcomes the requirement for CD4 help in exogenous antigen presentation to CD8+ T cells: implications for vaccine development in immune-deficient hosts
- PMID: 12782709
- PMCID: PMC2193905
- DOI: 10.1084/jem.20030109
Vaccine immunity to pathogenic fungi overcomes the requirement for CD4 help in exogenous antigen presentation to CD8+ T cells: implications for vaccine development in immune-deficient hosts
Abstract
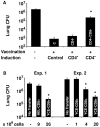
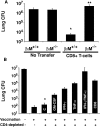
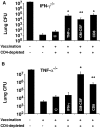
Systemic fungal infections with primary and opportunistic pathogens have become increasingly common and represent a growing health menace in patients with AIDS and other immune deficiencies. T lymphocyte immunity, in particular the CD4+ Th 1 cells, is considered the main defense against these pathogens, and their absence is associated with increased susceptibility. It would seem illogical then to propose vaccinating these vulnerable patients against fungal infections. We report here that CD4+ T cells are dispensable for vaccine-induced resistance against experimental fungal pulmonary infections with two agents, Blastomyces dermatitidis an extracellular pathogen, and Histoplasma capsulatum a facultative intracellular pathogen. In the absence of T helper cells, exogenous fungal antigens activated memory CD8+ cells in a major histocompatibility complex class I-restricted manner and CD8+ T cell-derived cytokines tumor necrosis factor alpha, interferon gamma, and granulocyte/macrophage colony-stimulating factor-mediated durable vaccine immunity. CD8+ T cells could also rely on alternate mechanisms for robust vaccine immunity, in the absence of some of these factors. Our results demonstrate an unexpected plasticity of immunity in compromised hosts at both the cellular and molecular level and point to the feasibility of developing vaccines against invasive fungal infections in patients with severe immune deficiencies, including those with few or no CD4+ T cells.
Figures



Comment in
-
Exploiting the redundancy in the immune system: vaccines can mediate protection by eliciting 'unnatural' immunity.J Exp Med. 2003 Jun 2;197(11):1401-4. doi: 10.1084/jem.20030637. J Exp Med. 2003. PMID: 12782708 Free PMC article. No abstract available.
Similar articles
-
Tc17 cells mediate vaccine immunity against lethal fungal pneumonia in immune deficient hosts lacking CD4+ T cells.PLoS Pathog. 2012;8(7):e1002771. doi: 10.1371/journal.ppat.1002771. Epub 2012 Jul 19. PLoS Pathog. 2012. PMID: 22829762 Free PMC article.
-
Progress in vaccination for histoplasmosis and blastomycosis: coping with cellular immunity.Med Mycol. 2005 Aug;43(5):381-9. doi: 10.1080/13693780500245875. Med Mycol. 2005. PMID: 16178365 Review.
-
IL-12 is required for induction but not maintenance of protective, memory responses to Blastomyces dermatitidis: implications for vaccine development in immune-deficient hosts.J Immunol. 2005 Oct 15;175(8):5288-97. doi: 10.4049/jimmunol.175.8.5288. J Immunol. 2005. PMID: 16210634
-
Requisite elements in vaccine immunity to Blastomyces dermatitidis: plasticity uncovers vaccine potential in immune-deficient hosts.J Immunol. 2002 Dec 15;169(12):6969-76. doi: 10.4049/jimmunol.169.12.6969. J Immunol. 2002. PMID: 12471131
-
Host response and Histoplasma capsulatum/macrophage molecular interactions.Med Mycol. 2000 Dec;38(6):399-406. doi: 10.1080/mmy.38.6.399.406. Med Mycol. 2000. PMID: 11204877 Review.
Cited by
-
Immunological correlates of protection mediated by a whole organism, Cryptococcus neoformans, vaccine deficient in chitosan.mBio. 2024 Aug 14;15(8):e0174624. doi: 10.1128/mbio.01746-24. Epub 2024 Jul 9. mBio. 2024. PMID: 38980038 Free PMC article.
-
CD28 is required for optimal induction, but not maintenance, of vaccine-induced immunity to Blastomyces dermatitidis.Infect Immun. 2005 Nov;73(11):7436-41. doi: 10.1128/IAI.73.11.7436-7441.2005. Infect Immun. 2005. PMID: 16239544 Free PMC article.
-
Vaccines for invasive fungal infections.F1000 Med Rep. 2011;3:13. doi: 10.3410/M3-13. Epub 2011 Jul 1. F1000 Med Rep. 2011. PMID: 21876719 Free PMC article.
-
Adaptive immunity to fungi.Cold Spring Harb Perspect Med. 2014 Nov 6;5(3):a019612. doi: 10.1101/cshperspect.a019612. Cold Spring Harb Perspect Med. 2014. PMID: 25377140 Free PMC article. Review.
-
Immunity to fungi and vaccine considerations.Cell Host Microbe. 2024 Oct 9;32(10):1681-1690. doi: 10.1016/j.chom.2024.09.011. Cell Host Microbe. 2024. PMID: 39389032 Review.
References
-
- Durden, F.M., and B. Elewski. 1997. Fungal infections in HIV-infected patients. Semin. Cutan. Med. Surg. 16:200–212. - PubMed
-
- Stansell, J.D. 1993. Pulmonary fungal infections in HIV-infected persons. Semin. Respir. Infect. 8:116–123. - PubMed
-
- Romani, L. 1997. The T cell response against fungal infections. Curr. Opin. Immunol. 9:484–490. - PubMed
-
- Conant, M.A. 1996. Fungal infections in immunocompromised individuals. Dermatol. Clin. 14:155–162. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

