Crystalline monoclonal antibodies for subcutaneous delivery
- PMID: 12782786
- PMCID: PMC165808
- DOI: 10.1073/pnas.1131899100
Crystalline monoclonal antibodies for subcutaneous delivery
Abstract
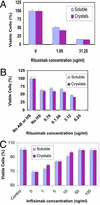
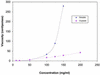
Therapeutic applications for mAbs have increased dramatically in recent years, but the large quantities required for clinical efficacy have limited the options that might be used for administration and thus have placed certain limitations on the use of these agents. We present an approach that allows for s.c. delivery of a small volume of a highly concentrated form of mAbs. Batch crystallization of three Ab-based therapeutics, rituximab, trastuzumab, and infliximab, provided products in high yield, with no detectable alteration to these proteins and with full retention of their biological activity in vitro. Administration s.c. of a crystalline preparation resulted in a remarkably long pharmacokinetic serum profile and a dose-dependent inhibition of tumor growth in nude mice bearing BT-474 xenografts (human breast cancer cells) in vivo. Overall, this approach of generating high-concentration, low-viscosity crystalline preparations of therapeutic Abs should lead to improved ease of administration and patient compliance, thus providing new opportunities for the biotechnology industry.
Figures




Similar articles
-
Micro-SPECT/CT with 111In-DTPA-pertuzumab sensitively detects trastuzumab-mediated HER2 downregulation and tumor response in athymic mice bearing MDA-MB-361 human breast cancer xenografts.J Nucl Med. 2009 Aug;50(8):1340-8. doi: 10.2967/jnumed.109.062224. Epub 2009 Jul 17. J Nucl Med. 2009. PMID: 19617342
-
Dual-labeled trastuzumab-based imaging agent for the detection of human epidermal growth factor receptor 2 overexpression in breast cancer.J Nucl Med. 2007 Sep;48(9):1501-10. doi: 10.2967/jnumed.107.042234. J Nucl Med. 2007. PMID: 17785729
-
On the selection of a tracer for PET imaging of HER2-expressing tumors: direct comparison of a 124I-labeled affibody molecule and trastuzumab in a murine xenograft model.J Nucl Med. 2009 Mar;50(3):417-25. doi: 10.2967/jnumed.108.057919. Epub 2009 Feb 17. J Nucl Med. 2009. PMID: 19223403
-
Monoclonal antibodies as targeted therapy in hematologic malignancies in older adults.Am J Geriatr Pharmacother. 2007 Sep;5(3):247-62. doi: 10.1016/j.amjopharm.2007.09.002. Am J Geriatr Pharmacother. 2007. PMID: 17996665 Review.
-
Role of intrathecal rituximab and trastuzumab in the management of leptomeningeal carcinomatosis.Ann Pharmacother. 2010 Oct;44(10):1633-40. doi: 10.1345/aph.1P197. Epub 2010 Aug 31. Ann Pharmacother. 2010. PMID: 20807868 Review.
Cited by
-
HSPiP, Computational, and Thermodynamic Model-Based Optimized Solvents for Subcutaneous Delivery of Tolterodine Tartrate and GastroPlus‑Based In Vivo Prediction in Humans: Part II.AAPS PharmSciTech. 2024 Jul 12;25(6):160. doi: 10.1208/s12249-024-02880-0. AAPS PharmSciTech. 2024. PMID: 38992299
-
Crystalline Antibody-Laden Alginate Particles: A Platform for Enabling High Concentration Subcutaneous Delivery of Antibodies.Adv Healthc Mater. 2023 Jun;12(15):e2202370. doi: 10.1002/adhm.202202370. Epub 2023 Feb 22. Adv Healthc Mater. 2023. PMID: 36745878 Free PMC article.
-
Structure-activity relationship for hydrophobic salts as viscosity-lowering excipients for concentrated solutions of monoclonal antibodies.Pharm Res. 2012 Nov;29(11):3102-9. doi: 10.1007/s11095-012-0802-9. Epub 2012 Jun 13. Pharm Res. 2012. PMID: 22692671
-
Metastability Gap in the Phase Diagram of Monoclonal IgG Antibody.Biophys J. 2017 Oct 17;113(8):1750-1756. doi: 10.1016/j.bpj.2017.08.048. Biophys J. 2017. PMID: 29045869 Free PMC article.
-
Low viscosity highly concentrated injectable nonaqueous suspensions of lysozyme microparticles.Langmuir. 2010 Jan 19;26(2):1067-74. doi: 10.1021/la9023426. Langmuir. 2010. PMID: 19803503 Free PMC article.
References
-
- Flieger, D., Renoth, S., Beier, I., Sauerbruch, T. & Schmidt-Wolf, I. (2000) Cell. Immunol. 204, 55–63. - PubMed
-
- Siegel, S. A., Shealy, D. J., Nakada, M. T., Le, J., Woulfe, D. S., Probert, L., Kollias, G., Ghrayeb, J., Vilcek, J. & Daddona, P. E. (1995) Cytokine 7, 15–25. - PubMed
-
- Pietras, R. J., Poen, J. C., Gallardo, D., Wongvipat, P. N., Lee, H. J. & Slamon, D. J. (1999) Cancer Res. 59, 1347–1355. - PubMed
-
- Brange, J. & Volund, A. (1999) Adv. Drug Delivery Rev. 35, 307–335. - PubMed
-
- Shenoy, B., Wang, Y., Shan, W. & Margolin, A. L. (2001) Biotechnol. Bioeng. 73, 358–369. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

