Dendritic-cell-specific ICAM3-grabbing non-integrin is essential for the productive infection of human dendritic cells by mosquito-cell-derived dengue viruses
- PMID: 12783086
- PMCID: PMC1326316
- DOI: 10.1038/sj.embor.embor866
Dendritic-cell-specific ICAM3-grabbing non-integrin is essential for the productive infection of human dendritic cells by mosquito-cell-derived dengue viruses
Abstract
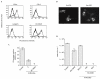
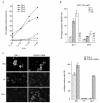
Dengue virus (DV) is a mosquito-borne flavivirus that causes haemorrhagic fever in humans. DV primarily targets immature dendritic cells (DCs) after a bite by an infected mosquito vector. Here, we analysed the interactions between DV and human-monocyte-derived DCs at the level of virus entry. We show that the DC-specific ICAM3-grabbing non-integrin (DC-SIGN) molecule, a cell-surface, mannose-specific, C-type lectin, binds mosquito-cell-derived DVs and allows viral replication. Conclusive evidence for the involvement of DC-SIGN in DV infection was obtained by the inhibition of viral infection by anti-DC-SIGN antibodies and by the soluble tetrameric ectodomain of DC-SIGN. Our data show that DC-SIGN functions as a DV-binding lectin by interacting with the DV envelope glycoprotein. Mosquito-cell-derived DVs may have differential infectivity for DC-SIGN-expressing cells. We suggest that the differential use of DC-SIGN by viral envelope glycoproteins may account for the immunopathogenesis of DVs.
Figures


References
-
- Bielefeldt-Ohmann H., Meyer M., Fitzpatrick D.R. & Mackenzie J.S. (2001) Dengue virus binding to human leukocyte cell lines: receptor usage differs between cell types and virus strains. Virus Res., 73, 81–89. - PubMed
-
- Chen Y., Maguire T., Hileman R.E., Fromm J.R., Esko J.D., Linhardt R.J. & Marks R.M. (1997) Dengue virus infectivity depends on envelope protein binding to target cell heparane sulfate. Nature Med., 3, 866–871. - PubMed
-
- Desprès P., Girard M. & Bouloy M. (1991) Characterization of yellow fever virus E and NS1 proteins expressed in Vero and Spodoptera frugiperda cells. J. Gen. Virol., 72, 1331–1342. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

