Six1 is required for the early organogenesis of mammalian kidney
- PMID: 12783782
- PMCID: PMC3872112
- DOI: 10.1242/dev.00536
Six1 is required for the early organogenesis of mammalian kidney
Abstract
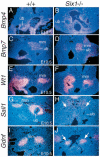
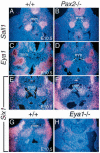
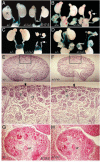
The murine Six gene family, homologous to Drosophila sine oculis (so) which encodes a homeodomain transcription factor, is composed of six members (Six1-6). Among the six members, only the Six2 gene has been previously shown to be expressed early in kidney development, but its function is unknown. We have recently found that the Six1 gene is also expressed in the kidney. In the developing kidney, Six1 is expressed in the uninduced metanephric mesenchyme at E10.5 and in the induced mesenchyme around the ureteric bud at E11.5. At E17.5 to P0, Six1 expression became restricted to a subpopulation of collecting tubule epithelial cells. To study its in vivo function, we have recently generated Six1 mutant mice. Loss of Six1 leads to a failure of ureteric bud invasion into the mesenchyme and subsequent apoptosis of the mesenchyme. These results indicate that Six1 plays an essential role in early kidney development. In Six1(-/-) kidney development, we have found that Pax2, Six2 and Sall1 expression was markedly reduced in the metanephric mesenchyme at E10.5, indicating that Six1 is required for the expression of these genes in the metanephric mesenchyme. In contrast, Eya1 expression was unaffected in Six1(-/-) metanephric mesenchyme at E10.5, indicating that Eya1 may function upstream of Six1. Moreover, our results show that both Eya1 and Six1 expression in the metanephric mesenchyme is preserved in Pax2(-/-) embryos at E10.5, further indicating that Pax2 functions downstream of Eya1 and Six1 in the metanephric mesenchyme. Thus, the epistatic relationship between Pax, Eya and Six genes in the metanephric mesenchyme during early kidney development is distinct from a genetic pathway elucidated in the Drosophila eye imaginal disc. Finally, our results show that Eya1 and Six1 genetically interact during mammalian kidney development, because most compound heterozygous embryos show hypoplastic kidneys. These analyses establish a role for Six1 in the initial inductive step for metanephric development.
Figures




References
-
- Al-Awqati Q, Oliver JA. Stem cells in the kidney. Kidney Int. 2002;61:387–395. - PubMed
-
- Ashley DJB, Mostofi FK. Renal agenesis and dysgenesis. J. Urol. 1960;83:211–230. - PubMed
-
- Brophy PD, Ostrom L, Lang KM, Dressler GR. Regulation of ureteric bud outgrowth by Pax2-dependent activation of the glial derived neurotrophic factor gene. Development. 2001;128:4747–4756. - PubMed
-
- Buller C, Xu X, Marquis V, Schwanke R, Xu P-X. Molecular effects of Eya1 domain mutations causing organ defects in BOR syndrome. Hum. Mol. Genet. 2001;10:2775–2781. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

