Control of landmark events in meiosis by the CDK Cdc28 and the meiosis-specific kinase Ime2
- PMID: 12783856
- PMCID: PMC196082
- DOI: 10.1101/gad.1101503
Control of landmark events in meiosis by the CDK Cdc28 and the meiosis-specific kinase Ime2
Abstract
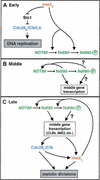
Meiosis is thought to require the protein kinase Ime2 early for DNA replication and the cyclin-dependent kinase Cdc28 late for chromosome segregation. To elucidate the roles of these kinases, we inhibited their activities early and late using conditional mutants that are sensitive to chemical inhibitors. Our studies reveal that both Cdc28 and Ime2 have critical roles in meiotic S phase and M phase. Early inhibition of analog-sensitive cdc28-as1 blocked DNA replication, revealing a previously undetected role for Cdc28. Yet Cdc28 was dispensable for one of its functions in the mitotic cell cycle, degradation of Sic1. Late addition of inhibitor to ime2-as1 revealed unexpected roles of Ime2 in the initiation and execution of chromosome segregation. The requirement of Ime2 for M phase is partially explained by its stimulation of the key meiotic transcription factor Ndt80, which is needed in turn for high Cdc28 activity. In accordance with a late role for Ime2, we observed an increase in its activity during M phase that depended on Cdc28 and Ndt80. We speculate that several unique features of the meiotic cell division reflect a division of labor and regulatory coordination between Ime2 and Cdc28.
Figures

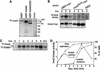




References
-
- Allers T. and Lichten, M. 2001. Differential timing and control of noncrossover and crossover recombination during meiosis. Cell 106: 47–57. - PubMed
-
- Bishop A.C., Kung, C.Y., Shah, K., Witucki, L., Shokat, K.M., and Liu, Y. 1999. Generation of monospecific nanomolar tyrosine kinase inhibitors via a chemical genetic approach. J. Am. Chem. Soc. 121: 627–631.
-
- Bishop A.C., Ubersax, J.A., Petsch, D.T., Matheos, D.P., Gray, N.S., Blethrow, J., Shimizu, E., Tsien, J.Z., Schultz, P.G., Rose, M.D., et al. 2000. A chemical switch for inhibitor-sensitive alleles of any protein kinase. Nature 407: 395–401. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
