Density of neoplastic lymphoid infiltrate, CD8+ T cells, and CD1a+ dendritic cells in mycosis fungoides
- PMID: 12783973
- PMCID: PMC1769963
- DOI: 10.1136/jcp.56.6.453
Density of neoplastic lymphoid infiltrate, CD8+ T cells, and CD1a+ dendritic cells in mycosis fungoides
Abstract
Background/aims: CD8+ T cells and epidermal/dermal dendritic cells expressing CD1a are found among neoplastic CD4+ T cells in mycosis fungoides (MF) lesions. This study analysed the relation of CD8+ tumour infiltrating lymphocytes (TILs), CD1a+ epidermal Langerhan's cells (LCs), and dermal dendritic cells (DDCs) to clinicopathological parameters in 46 MF cases.
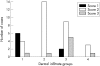
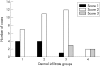
Methods: Pretreatment diagnostic biopsy specimens of 46 MF cases were submitted to histological analysis and immunohistochemistry. Four histological grades were defined based on the density of the neoplastic infiltrate: grade 1 (mild superficial perivascular infiltrate), grade 2 (moderate superficial perivascular infiltrate with some tendency to confluence), grade 3 (pronounced superficial band-like infiltrate), and grade 4 (deep nodular infiltrate). Epidermotropism was scored as low, moderate, or high. Numbers of CD8+ T cells and of dermal and epidermal CD1a+ cells were scored as 1 (low), 2 (moderate), and 3 (high). Correlations between these parameters and clinical data (age, sex, clinical type of lesions, stage, response to treatment, and recurrence) were analysed by the chi(2) test.
Results: Numbers of TILs and DDCs were associated with subepidermal infiltrates, being lower in less dense infiltrates, whereas there was no association between epidermal CD1a+ cells and the analysed parameters. Complete remission in treated patients was related to subepidermal infiltrates but not to TILs, LCs, or DDCs.
Conclusions: These results support the notion that CD8+ cells and dermal CD1a+ cells are active against tumour cells. MF with low numbers of TILs could represent an early stage of the disease, before TILs are activated against tumour specific antigens.
Figures

Similar articles
-
The cellular microenvironment and neoplastic population in mycosis fungoides skin lesions: a clinicopathological correlation.Eur J Dermatol. 2016 Dec 1;26(6):566-571. doi: 10.1684/ejd.2016.2847. Eur J Dermatol. 2016. PMID: 27545221
-
A subpopulation of Langerhans cells (CD1a+Lag-) increased in the dermis of plaque lesions of mycosis fungoides.J Am Acad Dermatol. 1991 Sep;25(3):491-9. doi: 10.1016/0190-9622(91)70229-u. J Am Acad Dermatol. 1991. PMID: 1717524
-
Dendritic cells and apoptosis in mycosis fungoides.Br J Dermatol. 2002 Dec;147(6):1171-9. doi: 10.1046/j.1365-2133.2002.04994.x. Br J Dermatol. 2002. PMID: 12452867
-
[Interstitial mycosis fungoid: a rare variant of mycosis fungoids. Two cases].Ann Pathol. 2011 Feb;31(1):36-40. doi: 10.1016/j.annpat.2010.09.003. Epub 2011 Jan 28. Ann Pathol. 2011. PMID: 21349387 Review. French.
-
Hypopigmented mycosis fungoides: a review of its clinical features and pathophysiology.An Bras Dermatol. 2013 Nov-Dec;88(6):954-60. doi: 10.1590/abd1806-4841.20132336. An Bras Dermatol. 2013. PMID: 24474105 Free PMC article. Review.
Cited by
-
OX40-OX40L Axis in Cutaneous T-Cell Lymphomas: Pathogenic, Prognostic, and Potential Therapeutic Perspectives.Biomolecules. 2025 May 13;15(5):715. doi: 10.3390/biom15050715. Biomolecules. 2025. PMID: 40427608 Free PMC article. Review.
-
Lesional skin chemokine CTACK/CCL27 expression in mycosis fungoides and disease control by IFN-alpha and PUVA therapy.Am J Transl Res. 2009 Jan 31;1(2):203-10. Am J Transl Res. 2009. PMID: 19956431 Free PMC article.
-
Multi-institutional Investigation: Circulating CD4:CD8 ratio is a prognosticator of response to total skin electron beam radiation in mycosis fungoides.Radiother Oncol. 2019 Feb;131:88-92. doi: 10.1016/j.radonc.2018.12.003. Epub 2018 Dec 31. Radiother Oncol. 2019. PMID: 30773193 Free PMC article.
-
Spatially Guided and Single Cell Tools to Map the Microenvironment in Cutaneous T-Cell Lymphoma.Cancers (Basel). 2023 Apr 18;15(8):2362. doi: 10.3390/cancers15082362. Cancers (Basel). 2023. PMID: 37190290 Free PMC article. Review.
-
Sézary Syndrome and Atopic Dermatitis: Comparison of Immunological Aspects and Targets.Biomed Res Int. 2016;2016:9717530. doi: 10.1155/2016/9717530. Epub 2016 May 17. Biomed Res Int. 2016. PMID: 27294147 Free PMC article. Review.
References
-
- Hansen ER. Immunoregulatory events in the skin of patients with cutaneous T-cell lymphoma. Arch Dermatol 1996;132:554–61. - PubMed
-
- Burg G, Dummer R, Haeffner A, et al. From inflammation to neoplasia: mycosis fungoides evolves from reactive inflammatory conditions (lymphoid infiltrates) transforming into neoplastic plaques and tumors. Arch Dermatol 2001;137:949–52. - PubMed
-
- Ralfkiaer E, Wantzin GL, Mason DY, et al. Phenotypic characterization of lymphocyte subsets in mycosis fungoides. Comparison with large plaque parapsoriasis and benign chronic dermatoses. Am J Clin Pathol 1985;84:610–19. - PubMed
-
- Ralfkiaer E. Immunohistological markers for the diagnosis of cutaneous lymphomas. Semin Diagn Pathol 1991;8:62–72. - PubMed
-
- Moll M, Reinhold U, Kukel S, et al. CD7-negative helper T cells accumulate in inflammatory skin lesions. J Invest Dermatol 1994;102:328–32. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
