Functional consequences of homo- but not hetero-oligomerization between transporters for the biogenic amine neurotransmitters
- PMID: 12787070
- PMCID: PMC3039113
- DOI: 10.1046/j.1471-4159.2003.01793.x
Functional consequences of homo- but not hetero-oligomerization between transporters for the biogenic amine neurotransmitters
Abstract
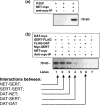
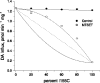
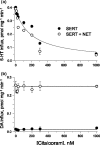
Before this study, the human norepinephrine transporter (hNET) was the only member of the biogenic amine neurotransmitter transporter family that had not been demonstrated to be a functional homo-oligomer. Here, using two forms of the transporter, I155C and hNET-myc, with distinct antigenicity and inhibitor sensitivity, we demonstrated that hNET exists as a homo-oligomer. hNET I155C is a functional mutant and is sensitive to inactivation by the sulfhydryl reagent [2-(trimethylammonium)ethyl]methanethiosulfonate, while hNET-myc is resistant to inactivation by this reagent. Coimmunoprecipitation of these two forms demonstrated that a physical interaction exists between norepinephrine transporter monomers. Further characterization of this physical interaction has revealed that the activity of norepinephrine transporters depends on interactions between monomers. Because norepinephrine transporters and serotonin transporters are the only two members of the neurotransmitter transporter family endogenously expressed in the cell membrane of the same cells, placental syncytiotrophoblasts, we tested the ability of norepinephrine transporters and serotonin transporters to associate and function in a hetero-oligomeric form. Similarly, coexpression of hNET-myc with serotonin transporter-FLAG showed a physical interaction in coimmunoprecipitation assays. However, coexpression of serotonin and norepinephrine transporters did not sensitize norepinephrine transporter activity to inhibition by citalopram, a selective serotonin transport inhibitor. Thus, the norepinephrine transporter-serotonin transporter physical association did not produce functional consequences. Based on this, we propose that the transporters for biogenic amine neurotransmitters interact functionally in homo- but not hetero-oligomeric forms.
Figures



Similar articles
-
Functional coupling of serotonin and noradrenaline transporters.J Neurochem. 2003 Aug;86(4):958-65. doi: 10.1046/j.1471-4159.2003.01899.x. J Neurochem. 2003. PMID: 12887693
-
Biogenic amine flux mediated by cloned transporters stably expressed in cultured cell lines: amphetamine specificity for inhibition and efflux.Mol Pharmacol. 1995 Mar;47(3):544-50. Mol Pharmacol. 1995. PMID: 7700252
-
Characteristics of drug interactions with recombinant biogenic amine transporters expressed in the same cell type.J Pharmacol Exp Ther. 1999 May;289(2):877-85. J Pharmacol Exp Ther. 1999. PMID: 10215666
-
Physiological genomics of antidepressant targets: keeping the periphery in mind.J Neurosci. 2001 Nov 1;21(21):8319-23. doi: 10.1523/JNEUROSCI.21-21-08319.2001. J Neurosci. 2001. PMID: 11606618 Free PMC article. Review.
-
Advances in sodium-ion coupled biogenic amine transporters.Life Sci. 1992;51(9):631-45. doi: 10.1016/0024-3205(92)90236-i. Life Sci. 1992. PMID: 1501510 Review.
Cited by
-
Molecular mechanisms of SERT in platelets: regulation of plasma serotonin levels.Mol Interv. 2010 Aug;10(4):231-41. doi: 10.1124/mi.10.4.6. Mol Interv. 2010. PMID: 20729489 Free PMC article. Review.
-
Dopamine transporter oligomerization: impact of combining protomers with differential cocaine analog binding affinities.J Neurochem. 2015 Apr;133(2):167-73. doi: 10.1111/jnc.13025. Epub 2015 Jan 26. J Neurochem. 2015. PMID: 25580950 Free PMC article.
-
Overview of the structure and function of the dopamine transporter and its protein interactions.Front Physiol. 2023 Mar 3;14:1150355. doi: 10.3389/fphys.2023.1150355. eCollection 2023. Front Physiol. 2023. PMID: 36935752 Free PMC article. Review.
-
At diabetes-like concentration, glucose down-regulates the placental serotonin transport system in a cell-cycle-dependent manner.J Neurochem. 2007 May;101(4):937-48. doi: 10.1111/j.1471-4159.2007.04469.x. Epub 2007 Mar 12. J Neurochem. 2007. Retraction in: J Neurochem. 2020 Apr;153(1):138. doi: 10.1111/jnc.14931. PMID: 17355243 Free PMC article. Retracted.
-
Immunomodulatory effects mediated by serotonin.J Immunol Res. 2015;2015:354957. doi: 10.1155/2015/354957. Epub 2015 Apr 19. J Immunol Res. 2015. PMID: 25961058 Free PMC article. Review.
References
-
- Amara S, Kuhar M. Neurotransmitter transporters — recent progress. Annu. Rev. Neurosci. 1993;16:73–93. - PubMed
-
- Balkovetz DF, Tirruppathi C, Leibach FH, Mahesh VB, Ganapathy V. Evidence for an imipramine-sensitive serotonin transporter in human placental brush-border membranes. J. Biol. Chem. 1989;264:2195–2198. - PubMed
-
- Bauman PA, Blakely RD. Determinants within the C-terminus of the human norepinephrine transporter dictate transporter trafficking, stability, and activity. Arch. Biochem. Biophys. 2002;404:80–91. - PubMed
-
- Blakely R, Berson H, Fremeau R, Caron M, Peek M, Prince H, Bradely C. Cloning and expression of a functional serotonin transporter from rat brain. Nature. 1991a;354:66–70. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

