Oligomerization and activation of the FliI ATPase central to bacterial flagellum assembly
- PMID: 12787361
- PMCID: PMC2528289
- DOI: 10.1046/j.1365-2958.2003.03506.x
Oligomerization and activation of the FliI ATPase central to bacterial flagellum assembly
Abstract
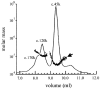
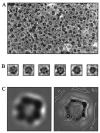
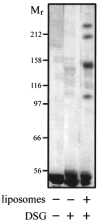
FliI is the peripheral membrane ATPase pivotal to the type III protein export mechanism underlying the assembly of the bacterial flagellum. Gel filtration and multiangle light scattering showed that purified soluble native FliI protein was in a monomeric state but, in the presence of ATP, FliI showed a propensity to oligomerize. Electron microscopy revealed that FliI assembles to a ring structure, the yield of which was increased by the presence of a non-hydrolysable ATP analogue. Single particle analysis of the resulting electron micrograph images, to which no symmetry was applied, showed that the FliI ring structure has sixfold symmetry and an external diameter of approximately 10 nm. The oligomeric ring has a central cavity of 2.5-3.0 nm, which is comparable to the known diameter of the flagellar export channel into which export substrates feed. Enzymatic activity of the FliI ATPase showed positive co-operativity, establishing that oligomerization and enzyme activity are coupled. Escherichia coli phospholipids increased enzyme co-operativity, and in vitro cross-linking demonstrated that they promoted FliI multimerization. The data reveal central facets of the structure and action of the flagellar assembly ATPase and, by extension, the homologous ATPases of virulence-related type III export systems.
Figures


References
-
- Aizawa SI. Bacterial flagella and type III secretion systems. FEMS Microbiol Lett. 2001;202:157–164. - PubMed
-
- Crowther RA, Henderson R, Smith JM. MRC image processing programs. J Struct Biol. 1996;116:9–16. - PubMed
-
- Davidson AL, Laghaeian SS, Mannering DE. The maltose transport system of Escherichia coli displays positive co-operativity in ATP hydrolysis. J Biol Chem. 1996;271:4858–4863. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

