Determination of the domain of the Lactobacillus delbrueckii subsp. bulgaricus cell surface proteinase PrtB involved in attachment to the cell wall after heterologous expression of the prtB gene in Lactococcus lactis
- PMID: 12788739
- PMCID: PMC161544
- DOI: 10.1128/AEM.69.6.3377-3384.2003
Determination of the domain of the Lactobacillus delbrueckii subsp. bulgaricus cell surface proteinase PrtB involved in attachment to the cell wall after heterologous expression of the prtB gene in Lactococcus lactis
Abstract
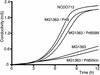
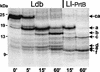
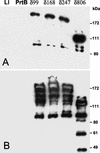
Belonging to the subtilase family, the cell surface proteinase (CSP) PrtB of Lactobacillus delbrueckii subsp. bulgaricus differs from other CSPs synthesized by lactic acid bacteria. Expression of the prtB gene under its own promoter was shown to complement the proteinase-deficient strain MG1363 (PrtP(-) PrtM(-)) of Lactococcus lactis subsp. cremoris. Surprisingly, the maturation process of PrtB, unlike that of lactococcal CSP PrtPs, does not require a specific PrtM-like chaperone. The carboxy end of PrtB was previously shown to be different from the consensus anchoring region of other CSPs and exhibits an imperfect duplication of 59 amino acids with a high lysine content. By using a deletion strategy, the removal of the last 99 amino acids, including the degenerated anchoring signal (LPKKT), was found to be sufficient to release a part of the truncated PrtB into the culture medium and led to an increase in PrtB activity. This truncated PrtB is still active and enables L. lactis MG1363 to grow in milk supplemented with glucose. By contrast, deletion of the last 806 amino acids of PrtB led to the secretion of an inactive proteinase. Thus, the utmost carboxy end of PrtB is involved in attachment to the bacterial cell wall. Proteinase PrtB constitutes a powerful tool for cell surface display of heterologous proteins like antigens.
Figures



Similar articles
-
A new cell surface proteinase: sequencing and analysis of the prtB gene from Lactobacillus delbruekii subsp. bulgaricus.J Bacteriol. 1996 Jun;178(11):3059-65. doi: 10.1128/jb.178.11.3059-3065.1996. J Bacteriol. 1996. PMID: 8655480 Free PMC article.
-
Lactobacillus bulgaricus proteinase expressed in Lactococcus lactis is a powerful carrier for cell wall-associated and secreted bovine beta-lactoglobulin fusion proteins.Appl Environ Microbiol. 2002 Jun;68(6):2917-23. doi: 10.1128/AEM.68.6.2917-2923.2002. Appl Environ Microbiol. 2002. PMID: 12039750 Free PMC article.
-
Display of heterologous proteins on the surface of Lactococcus lactis using the H and W domain of PrtB from Lactobacillus delburueckii subsp. bulgaricus as an anchoring matrix.J Appl Microbiol. 2008 Jun;104(6):1636-43. doi: 10.1111/j.1365-2672.2007.03690.x. Epub 2008 Feb 19. J Appl Microbiol. 2008. PMID: 18298534
-
Multi-domain, cell-envelope proteinases of lactic acid bacteria.Antonie Van Leeuwenhoek. 1999 Jul-Nov;76(1-4):139-55. Antonie Van Leeuwenhoek. 1999. PMID: 10532376 Review.
-
Regulation of antimicrobial peptide production by autoinducer-mediated quorum sensing in lactic acid bacteria.Antonie Van Leeuwenhoek. 2002 Aug;82(1-4):133-45. Antonie Van Leeuwenhoek. 2002. PMID: 12369185 Review.
Cited by
-
Dissimilar properties of two recombinant Lactobacillus acidophilus strains displaying Salmonella FliC with different anchoring motifs.Appl Environ Microbiol. 2011 Sep;77(18):6587-96. doi: 10.1128/AEM.05153-11. Epub 2011 Jul 22. Appl Environ Microbiol. 2011. PMID: 21784918 Free PMC article.
-
Group-specific comparison of four lactobacilli isolated from human sources using differential blast analysis.Genes Nutr. 2011 Aug;6(3):319-40. doi: 10.1007/s12263-010-0191-9. Epub 2010 Oct 28. Genes Nutr. 2011. PMID: 21484153 Free PMC article.
-
A multifunction ABC transporter (Opt) contributes to diversity of peptide uptake specificity within the genus Lactococcus.J Bacteriol. 2004 Oct;186(19):6492-500. doi: 10.1128/JB.186.19.6492-6500.2004. J Bacteriol. 2004. PMID: 15375130 Free PMC article.
-
Role of the Sortase A in the Release of Cell-Wall Proteinase PrtS in the Growth Medium of Streptococcus thermophilus 4F44.Microorganisms. 2021 Nov 18;9(11):2380. doi: 10.3390/microorganisms9112380. Microorganisms. 2021. PMID: 34835505 Free PMC article.
References
-
- Bernasconi, E., J. E. Germond, M. Delley, R. Fritsche, and B. Corthesy. 2002. Lactobacillus bulgaricus proteinase expressed in Lactococcus lactis is a powerful carrier for cell wall-associated and secreted bovine beta-lactoglobulin fusion proteins. Appl. Environ. Microbiol. 68:2917-2923. - PMC - PubMed
-
- Bethe, G., R. Nau, A. Wellmer, R. Hakenbeck, R. R. Reinert, H. P. Heinz, and G. Zysk. 2001. The cell wall-associated serine protease PrtA: a highly conserved virulence factor of Streptococcus pneumoniae. FEMS Microbiol. Lett. 205:99-104. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

