Mice lacking phosphatidylinositol transfer protein-alpha exhibit spinocerebellar degeneration, intestinal and hepatic steatosis, and hypoglycemia
- PMID: 12788952
- PMCID: PMC7798478
- DOI: 10.1074/jbc.M303591200
Mice lacking phosphatidylinositol transfer protein-alpha exhibit spinocerebellar degeneration, intestinal and hepatic steatosis, and hypoglycemia
Abstract
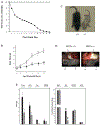
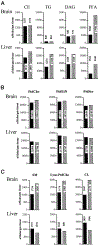
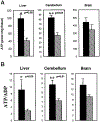
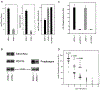
Phosphatidylinositol transfer proteins (PITPs) regulate the interface between lipid metabolism and cellular functions. We now report that ablation of PITP alpha function leads to aponecrotic spinocerebellar disease, hypoglycemia, and intestinal and hepatic steatosis in mice. The data indicate that hypoglycemia is in part associated with reduced proglucagon gene expression and glycogenolysis that result from pancreatic islet cell defects. The intestinal and hepatic steatosis results from the intracellular accumulation of neutral lipid and free fatty acid mass in these organs and suggests defective trafficking of triglycerides and diacylglycerols from the endoplasmic reticulum. We propose that deranged intestinal and hepatic lipid metabolism and defective proglucagon gene expression contribute to hypoglycemia in PITP alpha-/- mice, and that hypoglycemia is a significant contributing factor in the onset of spinocerebellar disease. Taken together, the data suggest an unanticipated role for PITP alpha in with glucose homeostasis and in mammalian endoplasmic reticulum functions that interface with transport of specific luminal lipid cargoes.
Figures






References
-
- Cleves AE, McGee TP, and Bankaitis VA (1991) Trends Cell Biol. 1, 30–34 - PubMed
-
- Wirtz KWA (1991) Annu. Rev. Biochem 60, 73–99 - PubMed
-
- Bankaitis VA, Aitken JR, Cleves AE, and Dowhan W (1990) Nature 347, 561–562 - PubMed
-
- Kearns BG, Alb JG Jr., and Bankaitis VA (1998) Trends Cell Biol. 8, 276–282 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

