The complete genome sequence of Mycobacterium bovis
- PMID: 12788972
- PMCID: PMC164681
- DOI: 10.1073/pnas.1130426100
The complete genome sequence of Mycobacterium bovis
Abstract
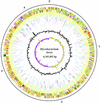
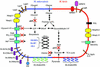
Mycobacterium bovis is the causative agent of tuberculosis in a range of animal species and man, with worldwide annual losses to agriculture of $3 billion. The human burden of tuberculosis caused by the bovine tubercle bacillus is still largely unknown. M. bovis was also the progenitor for the M. bovis bacillus Calmette-Guérin vaccine strain, the most widely used human vaccine. Here we describe the 4,345,492-bp genome sequence of M. bovis AF2122/97 and its comparison with the genomes of Mycobacterium tuberculosis and Mycobacterium leprae. Strikingly, the genome sequence of M. bovis is >99.95% identical to that of M. tuberculosis, but deletion of genetic information has led to a reduced genome size. Comparison with M. leprae reveals a number of common gene losses, suggesting the removal of functional redundancy. Cell wall components and secreted proteins show the greatest variation, indicating their potential role in host-bacillus interactions or immune evasion. Furthermore, there are no genes unique to M. bovis, implying that differential gene expression may be the key to the host tropisms of human and bovine bacilli. The genome sequence therefore offers major insight on the evolution, host preference, and pathobiology of M. bovis.
Figures
References
-
- Krebs, J. (1997) Bovine Tuberculosis in Cattle and Badgers: Report to the Rt. Hon. Dr. Jack Cunningham MP by the Independent Scientific Review Group (Department for Environment, Food and Rural Affairs, London).
-
- Weyer, K., Fourie, P. B., Durrheim, D., Lancaster, J., Haslov, K. & Bryden, H. (1999) Int. J. Tuberc. Lung Dis. 3, 1113-1119. - PubMed
-
- Morris, R. & Pfeiffer, D. (1995) N. Zealand Vet. J. 43, 256-265. - PubMed
-
- Payeur, J. B., Church, S., Mosher, L., Robinson-Dunn, B., Schmitt, S. & Whipple, D. (2002) Ann. N.Y. Acad. Sci. 969, 259-261. - PubMed
-
- Cole, S. T., Brosch, R., Parkhill, J., Garnier, T., Churcher, C., Harris, D., Gordon, S. V., Eiglmeier, K., Gas, S., Barry, C. E., 3rd, et al. (1998) Nature 393, 537-544. - PubMed
Publication types
MeSH terms
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

