Maspin expression inhibits osteolysis, tumor growth, and angiogenesis in a model of prostate cancer bone metastasis
- PMID: 12788977
- PMCID: PMC164676
- DOI: 10.1073/pnas.1331360100
Maspin expression inhibits osteolysis, tumor growth, and angiogenesis in a model of prostate cancer bone metastasis
Abstract
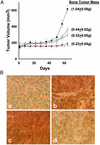
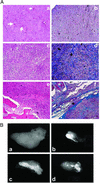
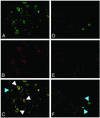
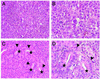
Emerging evidence indicates that tumor-associated proteolytic remodeling of bone matrix may underlie the capacity of tumor cells to colonize and survive in the bone microenvironment. Of particular importance, urokinase-type plasminogen activator (uPA) has been shown to correlate with human prostate cancer (PC) metastasis. The importance of this protease may be related to its ability to initiate a proteolytic cascade, leading to the activation of multiple proteases and growth factors. Previously, we showed that maspin, a serine protease inhibitor, specifically inhibits PC-associated uPA and PC cell invasion and motility in vitro. In this article, we showed that maspin-expressing transfectant cells derived from PC cell line DU145 were inhibited in in vitro extracellular matrix and collagen degradation assays. To test the effect of tumor-associated maspin on PC-induced bone matrix remodeling and tumor growth, we injected the maspin-transfected DU145 cells into human fetal bone fragments, which were previously implanted in immunodeficient mice. These studies showed that maspin expression decreased tumor growth, reduced osteolysis, and decreased angiogenesis. Furthermore, the maspin-expressing tumors contained significant fibrosis and collagen staining, and exhibited a more glandular organization. These data represent evidence that maspin inhibits PC-induced bone matrix remodeling and induces PC glandular redifferentiation. These results support our current working hypothesis that maspin exerts its tumor suppressive role, at least in part, by blocking the pericellular uPA system and suggest that maspin may offer an opportunity to improve therapeutic intervention of bone metastasis.
Figures
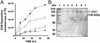
References
-
- Tu, S. M., Millikan, R. E., Mengistu, B., Delpassand, E. S., Amato, R. J., Pagliaro, L. C., Daliani, D., Papandreou, C. N., Smith, T. L., Kim, J., et al. (2001) Lancet 357, 336-341. - PubMed
-
- Shimazaki, J., Higa, T., Akimoto, S., Masai, M. & Isaka, S. (1992) Adv. Exp. Med. Biol. 324, 269-275. - PubMed
-
- Akimoto, S., Furuya, Y., Akakura, K. & Ito, H. (1998) Endocr. J. 45, 97-104. - PubMed
-
- Revilla, M., Arribas, I., Sanchez, C. M., Villa, L. F., Bethencourt, F. & Rico, H. (1998) Prostate 35, 243-247. - PubMed
-
- Rubens, R. D. (1998) Eur. J. Cancer 34, 210-213. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

