Effectiveness of neuraminidase inhibitors in treatment and prevention of influenza A and B: systematic review and meta-analyses of randomised controlled trials
- PMID: 12791735
- PMCID: PMC161558
- DOI: 10.1136/bmj.326.7401.1235
Effectiveness of neuraminidase inhibitors in treatment and prevention of influenza A and B: systematic review and meta-analyses of randomised controlled trials
Abstract
Objective: To review the clinical effectiveness of oseltamivir and zanamivir for the treatment and prevention of influenza A and B.
Design: Systematic review and meta-analyses of randomised controlled trials.
Data sources: Published studies were retrieved from electronic bibliographic databases; supplementary data were obtained from the manufacturers.
Selection of studies: Randomised controlled, double blind trials that were published in English, had data available before 31 December 2001, evaluated treatment or prevention of naturally occurring influenza with zanamivir or oseltamivir (if given using the formulation and dosage licensed for clinical use), and reported at least one end point of relevance.
Review methods: The main outcome measures were the median time to the alleviation of symptoms (for treatment trials) and number of flu episodes avoided (for prevention trials). Three population groups were defined: children aged 12 years and under; otherwise healthy individuals aged 12 to 65 years; and "high risk" individuals (those with certain chronic medical conditions or aged 65 years and older).
Results: Seventeen treatment trials and seven prevention trials identified met the inclusion criteria. All trials included compared one of the drugs against placebo or standard care. Treatment of children, otherwise healthy individuals, and high risk populations with zanamivir reduced the median duration of symptoms in days respectively by 1.0 (95% confidence interval 0.5 to 1.5), 0.8 (0.3 to 1.3), and 0.9 (-0.1 to 1.9) for the intention to treat population. The corresponding results, in days, for oseltamivir were 0.9 (0.3 to 1.5), 0.9 (0.3 to 1.4), and 0.4 (-0.7 to 1.4). The effect of giving zanamivir and oseltamivir prophylactically resulted in a relative reduction of 70-90% in the odds of developing flu, depending on the strategy adopted and the population studied.
Conclusions: Evidence from randomised controlled trials consistently supports the view that both oseltamivir and zanamivir are clinically effective for treating and preventing flu. However, evidence is limited for the treatment of certain populations and for all prevention strategies.
Figures

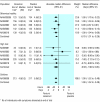
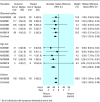
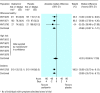

Comment in
-
Preventing and treating influenza.BMJ. 2003 Jun 7;326(7401):1223-4. doi: 10.1136/bmj.326.7401.1223. BMJ. 2003. PMID: 12791711 Free PMC article. No abstract available.
-
Neuraminidase inhibitors for influenza A and B: study showed benefits of treatment are marginal.BMJ. 2003 Jul 12;327(7406):105. doi: 10.1136/bmj.327.7406.105-a. BMJ. 2003. PMID: 12855537 Free PMC article. No abstract available.
-
Neuraminidase inhibitors for influenza A and B: antivirals need to be protected from adverse conditions to retain effectiveness.BMJ. 2003 Jul 12;327(7406):105-6. doi: 10.1136/bmj.327.7406.105-c. BMJ. 2003. PMID: 12855538 Free PMC article. No abstract available.
-
Neuraminidase inhibitors for influenza A and B: PROSE may be as useful as POEMs.BMJ. 2003 Jul 12;327(7406):105. doi: 10.1136/bmj.327.7406.105-b. BMJ. 2003. PMID: 12855540 Free PMC article. No abstract available.
-
Guidelines on neuraminidase inhibitors in children are not supported by evidence.BMJ. 2004 Jan 24;328(7433):227. doi: 10.1136/bmj.328.7433.227. BMJ. 2004. PMID: 14739201 Free PMC article. No abstract available.
References
-
- Nicholson KG, Webster RG, Hay AJ. Textbook of influenza. Oxford: Blackwell Science, 1998.
-
- Cochran K, Maasasab H, Tsunoda A, Berlin BS. Studies on the antiviral activity of amantadine hydrochloride. Ann N Y Acad Sci 1965;130: 432-9. - PubMed
-
- Tsunoda A, Maasab HF, Cochran KW, Eveland WC. Antiviral activity of alpha-methyl-1-admantanemethylamine hydrochloride. Antimicrobial Agents Chemother 1965;5: 553-60. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
