Diagnostic potential of parechovirus capsid proteins
- PMID: 12791839
- PMCID: PMC156510
- DOI: 10.1128/JCM.41.6.2294-2299.2003
Diagnostic potential of parechovirus capsid proteins
Abstract
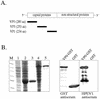
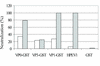
To study humoral and cellular immunity against human parechovirus type 1 (HPEV1), the viral capsid proteins VP0, VP1, and VP3 were expressed and purified as glutathione S-transferase (GST)-tagged recombinant proteins. The fusion proteins were used to raise antisera in rabbits. VP0 and VP1 antisera specifically detected HPEV1-infected cells in culture by immunoperoxidase staining and immunofluorescence. Furthermore, antisera against the VP0 and VP1 proteins had neutralizing effects against HPEV1 infection. When the HPEV1 antibody titers of 20 adults and 55 children were determined by a microneutralization test, the prevalence of HPEV1 antibodies in the adult population was 96%, while 50% of children were seropositive. Selected sera were used to evaluate HPEV1 fusion proteins as antigens in an enzyme immunoassay. The VP3 capsid protein appeared to be suitable for the purpose, with specificity of 100% and sensitivity of 96% compared to the neutralization test. Furthermore, T-cell responses to the purified HPEV1 and HPEV1 capsid fusion proteins were studied in 20 adults. Sixty percent of the subjects had T-cell proliferation responses to purified HPEV1, and 90% of the subjects also had positive T-cell responses to at least one of the GST capsid proteins.
Figures


Similar articles
-
Human Memory B Cells Producing Potent Cross-Neutralizing Antibodies against Human Parechovirus: Implications for Prevalence, Treatment, and Diagnosis.J Virol. 2015 Aug;89(15):7457-64. doi: 10.1128/JVI.01079-15. Epub 2015 May 6. J Virol. 2015. PMID: 25948742 Free PMC article.
-
Antigenic properties of human parechovirus 1.J Gen Virol. 2000 Jul;81(Pt 7):1709-18. doi: 10.1099/0022-1317-81-7-1709. J Gen Virol. 2000. PMID: 10859376
-
Characterization of polyclonal antibodies against the capsid proteins of Ljungan virus.J Virol Methods. 2008 Jun;150(1-2):34-40. doi: 10.1016/j.jviromet.2008.02.012. Epub 2008 Apr 9. J Virol Methods. 2008. PMID: 18403027
-
VP1 pseudocapsids, but not a glutathione-S-transferase VP1 fusion protein, prevent polyomavirus infection in a T-cell immune deficient experimental mouse model.J Med Virol. 2003 Jun;70(2):293-300. doi: 10.1002/jmv.10394. J Med Virol. 2003. PMID: 12696121
-
Parechoviruses, a novel group of human picornaviruses.Ann Med. 2001 Oct;33(7):466-71. doi: 10.3109/07853890109002095. Ann Med. 2001. PMID: 11680794 Review.
Cited by
-
Human Parechovirus: an Increasingly Recognized Cause of Sepsis-Like Illness in Young Infants.Clin Microbiol Rev. 2017 Nov 15;31(1):e00047-17. doi: 10.1128/CMR.00047-17. Print 2018 Jan. Clin Microbiol Rev. 2017. PMID: 29142080 Free PMC article. Review.
-
Development of a sensitive and specific epitope-blocking ELISA for universal detection of antibodies to human enterovirus 71 strains.PLoS One. 2013;8(1):e55517. doi: 10.1371/journal.pone.0055517. Epub 2013 Jan 31. PLoS One. 2013. PMID: 23383215 Free PMC article.
-
The Structure of Human Parechovirus 1 Reveals an Association of the RNA Genome with the Capsid.J Virol. 2015 Nov 18;90(3):1377-86. doi: 10.1128/JVI.02346-15. Print 2016 Feb 1. J Virol. 2015. PMID: 26581987 Free PMC article.
-
Parechovirus A Pathogenesis and the Enigma of Genotype A-3.Viruses. 2019 Nov 14;11(11):1062. doi: 10.3390/v11111062. Viruses. 2019. PMID: 31739613 Free PMC article. Review.
-
Human Memory B Cells Producing Potent Cross-Neutralizing Antibodies against Human Parechovirus: Implications for Prevalence, Treatment, and Diagnosis.J Virol. 2015 Aug;89(15):7457-64. doi: 10.1128/JVI.01079-15. Epub 2015 May 6. J Virol. 2015. PMID: 25948742 Free PMC article.
References
-
- Cello, J., O. Strannegard, and B. Svennerholm. 1996. A study of the cellular immune response to enteroviruses in humans: identification of cross-reactive T cell epitopes on the structural proteins of enteroviruses. J. Gen. Virol. 77:2097-2108. - PubMed
-
- Ghazi, F., P. Hughes, T. Hyypiä, and G. Stanway. 1998. Molecular analysis of human parechovirus type 2 (formerly echovirus 23). J. Gen. Virol. 79:2641-2650. - PubMed
-
- Grist, N., E. J. Bell, and F. Assaad. 1978. Enteroviruses in human disease. Prog. Med. Virol. 24:114-157. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

