Development of group- and serotype-specific one-step SYBR green I-based real-time reverse transcription-PCR assay for dengue virus
- PMID: 12791857
- PMCID: PMC156548
- DOI: 10.1128/JCM.41.6.2408-2416.2003
Development of group- and serotype-specific one-step SYBR green I-based real-time reverse transcription-PCR assay for dengue virus
Abstract
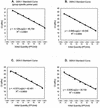
A quantitative one-step SYBR Green I-based reverse transcription (RT)-PCR system was developed for the detection and differentiation of four different dengue virus serotypes in acute-phase serum samples. A set of group- and serotype-specific primer pairs was designed against conserved sequences in the core region and evaluated for clinical diagnosis. A linear relationship was obtained between the amount of input RNA and cycle threshold (Ct) value over a range of 10 to 10(7) PFU per ml of cell culture-derived dengue viruses. The detection limit of the group-specific primer pair was between 4.1 and 43.5 PFU/ml for four dengue serotypes. The detection limit of each of the serotype-specific primer pairs was calculated to be 10 PFU/ml for dengue virus serotype 1 (DEN-1), 4.6 PFU/ml for DEN-2, 4.1 PFU/ml for DEN-3, and 5 PFU/ml for DEN-4. Comparisons between the one-step SYBR Green-based RT-PCR assay and the conventional cell culture method in the clinical diagnosis of dengue virus infection from acute-phase serum samples of confirmed dengue patients were performed. The results showed that 83 and 67% of 193 acute-phase serum samples tested were positive by the one-step SYBR Green-based RT-PCR method and cell culture method, respectively. Further analysis showed that the one-step SYBR Green-based RT-PCR method could detect twice as many acute-phase serum samples with positive dengue-specific immunoglobulin M (IgM) and/or IgG antibodies than cell culture method. Our results demonstrate the potential clinical application of the one-step SYBR Green I-based RT-PCR assay for the detection and differentiation of dengue virus RNA.
Figures



References
-
- Alcon, S., A. Talarmin, M. Debruyne, A. Falconar, V. Deubel, and M. Flamand. 2002. Enzyme-linked immunosorbent assay to dengue virus type 1 nonstructural protein NS1 reveals circulation of the antigen in the blood during the acute phase of disease in patients experiencing primary or secondary infections. J. Clin. Microbiol. 40:376-381. - PMC - PubMed
-
- Burke, D. S., A. Nisalak, D. E. Johnson, and R. M. Scott. 1988. A prospective study of dengue infections in Bangkok. Am. J. Trop. Med. Hyg. 38:172-180. - PubMed
-
- Callahan, J. D., S. J. Wu, A. Dion-Schultz, B. E. Mangold, L. F. Peruski, D. M. Watts, K. R. Porter, G. R. Murpgy, W. Suharyono, C. C. King, C. G. Hayes, and J. J. Temenak. 2001. Development and evaluation of serotype- and group-specific fluorogenic reverse transcriptase PCR (TaqMan) assays for dengue virus. J. Clin. Microbiol. 39:4119-4124. - PMC - PubMed
-
- Deubel, V., R. M. Kinney, and D. W. Trent. 1988. Nucleotide sequence and deduced amino acid sequence of the nonstructural proteins of dengue type 2 virus, Jamaica genotype: comparative analysis of the full-length genome. Virology 165:234-244. - PubMed
-
- Drosten, C., S. Gottig, S. Schilling, M. Asper, M. Panning, H. Schmitz, and S. Gunther. 2002. Rapid detection and quantitation of RNA of Ebola and Marburg viruses, Lassa virus, Crimean-Congo hemorrhagic fever virus, Rift valley fever virus, dengue virus, and Yellow fever virus by real-time reverse transcription-PCR. J. Clin. Microbiol. 40:2323-2330. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

