A simple energy-conserving system: proton reduction coupled to proton translocation
- PMID: 12792025
- PMCID: PMC164623
- DOI: 10.1073/pnas.1331436100
A simple energy-conserving system: proton reduction coupled to proton translocation
Abstract
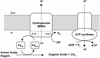
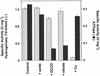
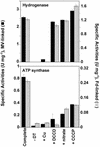
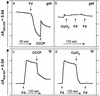
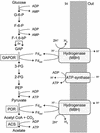
Oxidative phosphorylation involves the coupling of ATP synthesis to the proton-motive force that is generated typically by a series of membrane-bound electron transfer complexes, which ultimately reduce an exogenous terminal electron acceptor. This is not the case with Pyrococcus furiosus, an archaeon that grows optimally near 100 degrees C. It has an anaerobic respiratory system that consists of a single enzyme, a membrane-bound hydrogenase. Moreover, it does not require an added electron acceptor as the enzyme reduces protons, the simplest of acceptors, to hydrogen gas by using electrons from the cytoplasmic redox protein ferredoxin. It is demonstrated that the production of hydrogen gas by membrane vesicles of P. furiosus is directly coupled to the synthesis of ATP by means of a proton-motive force that has both electrochemical and pH components. Such a respiratory system enables rationalization in this organism of an unusual glycolytic pathway that was previously thought not to conserve energy. It is now clear that the use of ferredoxin in place of the expected NAD as the electron acceptor for glyceraldehyde 3-phosphate oxidation enables energy to be conserved by hydrogen production. In addition, this simple respiratory mechanism readily explains why the growth yields of P. furiosus are much higher than could be accounted for if ATP synthesis occurred only by substrate-level phosphorylation. The ability of microorganisms such as P. furiosus to couple hydrogen production to energy conservation has important ramifications not only in the evolution of respiratory systems but also in the origin of life itself.
Figures

Similar articles
-
Evolution of membrane bioenergetics.J Supramol Struct. 1980;13(4):421-46. doi: 10.1002/jss.400130403. J Supramol Struct. 1980. PMID: 6453255 Review.
-
Reinvestigation of the steady-state kinetics and physiological function of the soluble NiFe-hydrogenase I of Pyrococcus furiosus.J Bacteriol. 2008 Mar;190(5):1584-7. doi: 10.1128/JB.01562-07. Epub 2007 Dec 21. J Bacteriol. 2008. PMID: 18156274 Free PMC article.
-
Energy conservation via electron bifurcating ferredoxin reduction and proton/Na(+) translocating ferredoxin oxidation.Biochim Biophys Acta. 2013 Feb;1827(2):94-113. doi: 10.1016/j.bbabio.2012.07.002. Epub 2012 Jul 16. Biochim Biophys Acta. 2013. PMID: 22800682 Review.
-
Proton translocation in methanogens.Methods Enzymol. 2011;494:257-80. doi: 10.1016/B978-0-12-385112-3.00013-5. Methods Enzymol. 2011. PMID: 21402219
-
Proton-coupled electron transfer dynamics in the catalytic mechanism of a [NiFe]-hydrogenase.J Am Chem Soc. 2015 Apr 8;137(13):4558-66. doi: 10.1021/jacs.5b01791. Epub 2015 Mar 30. J Am Chem Soc. 2015. PMID: 25790178
Cited by
-
The Rnf complex is a Na+ coupled respiratory enzyme in a fermenting bacterium, Thermotoga maritima.Commun Biol. 2020 Aug 7;3(1):431. doi: 10.1038/s42003-020-01158-y. Commun Biol. 2020. PMID: 32770029 Free PMC article.
-
Assembly of Natively Synthesized Dual Chromophores Into Functional Actinorhodopsin.Front Microbiol. 2021 Apr 28;12:652328. doi: 10.3389/fmicb.2021.652328. eCollection 2021. Front Microbiol. 2021. PMID: 33995310 Free PMC article.
-
Metabolic energy-based modelling explains product yielding in anaerobic mixed culture fermentations.PLoS One. 2015 May 18;10(5):e0126739. doi: 10.1371/journal.pone.0126739. eCollection 2015. PLoS One. 2015. PMID: 25992959 Free PMC article.
-
Horizontal transfer of two operons coding for hydrogenases between bacteria and archaea.J Mol Evol. 2005 May;60(5):557-65. doi: 10.1007/s00239-004-0094-8. J Mol Evol. 2005. PMID: 15983865
-
Thermococcus indicus sp. nov., a Fe(III)-reducing hyperthermophilic archaeon isolated from the Onnuri Vent Field of the Central Indian Ocean ridge.J Microbiol. 2020 Apr;58(4):260-267. doi: 10.1007/s12275-020-9424-9. Epub 2020 Apr 1. J Microbiol. 2020. PMID: 32239454
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

