Possible role of membrane gamma-glutamyltransferase activity in the facilitation of transferrin-dependent and -independent iron uptake by cancer cells
- PMID: 12793906
- PMCID: PMC162169
- DOI: 10.1186/1475-2867-3-7
Possible role of membrane gamma-glutamyltransferase activity in the facilitation of transferrin-dependent and -independent iron uptake by cancer cells
Abstract
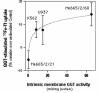
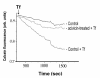
BACKGROUND: The molecular mechanisms by which iron is physiologically transported trough the cellular membranes are still only partially understood. Several studies indicate that a reduction step of ferric iron to ferrous is necessary, both in the case of transferrin-mediated and transferrin-independent iron uptake. Recent studies from our laboratory described gamma-glutamyltransferase activity (GGT) as a factor capable to effect iron reduction in the cell microenvironment. GGT is located on the outer aspect of plasma membrane of most cell types, and is often expressed at high levels in malignant tumors and their metastases. The present study was aimed at verifying the possibility that GGT-mediated iron reduction may participate in the process of cellular iron uptake. RESULTS: Four distinct human tumor cell lines, exhibiting different levels of GGT activity, were studied. The uptake of transferrin-bound iron was investigated by using 55Fe-loaded transferrin, as well as by monitoring fluorimetrically the intracellular iron levels in calcein-preloaded cells. Transferrin-independent iron uptake was investigated using 55Fe complexed by nitrilotriacetic acid (55Fe-NTA complex).The stimulation of GGT activity, by administration to cells of the substrates glutathione and glycyl-glycine, was generally reflected in a facilitation of transferrin-bound iron uptake. The extent of such facilitation was correlated with the intrinsic levels of the enzyme present in each cell line. Accordingly, inhibition of GGT activity by means of two independent inhibitors, acivicin and serine/boric acid complex, resulted in a decreased uptake of transferrin-bound iron. With Fe-NTA complex, the inhibitory effect - but not the stimulatory one - was also observed. CONCLUSION: It is concluded that membrane GGT can represent a facilitating factor in iron uptake by GGT-expressing cancer cells, thus providing them with a selective growth advantage over clones that do not possess the enzyme.
Figures


Similar articles
-
Plasma membrane gamma-glutamyltransferase activity facilitates the uptake of vitamin C in melanoma cells.Free Radic Biol Med. 2004 Dec 1;37(11):1906-15. doi: 10.1016/j.freeradbiomed.2004.08.015. Free Radic Biol Med. 2004. PMID: 15528049
-
gamma-Glutamyl transpeptidase-dependent lipid peroxidation in isolated hepatocytes and HepG2 hepatoma cells.Free Radic Biol Med. 1997;22(5):853-60. doi: 10.1016/s0891-5849(96)00422-4. Free Radic Biol Med. 1997. PMID: 9119254
-
Reduction site of transferrin-dependent and transferrin-independent iron in cultured human fibroblasts.J Biochem. 1994 May;115(5):849-52. doi: 10.1093/oxfordjournals.jbchem.a124428. J Biochem. 1994. PMID: 7961596
-
Brain iron homeostasis.Dan Med Bull. 2002 Nov;49(4):279-301. Dan Med Bull. 2002. PMID: 12553165 Review.
-
Iron absorption and transport.Am J Med Sci. 1999 Oct;318(4):213-29. doi: 10.1097/00000441-199910000-00002. Am J Med Sci. 1999. PMID: 10522550 Review.
Cited by
-
Endothelial γ-glutamyltransferase contributes to the vasorelaxant effect of S-nitrosoglutathione in rat aorta.PLoS One. 2012;7(9):e43190. doi: 10.1371/journal.pone.0043190. Epub 2012 Sep 11. PLoS One. 2012. PMID: 22984412 Free PMC article.
-
Effects of various interventions on the occurrence of macrovascular invasion of hepatocellular carcinoma after the baseline serum γ-glutamyltransferase stratification.Onco Targets Ther. 2019 Feb 28;12:1671-1679. doi: 10.2147/OTT.S184302. eCollection 2019. Onco Targets Ther. 2019. PMID: 30881022 Free PMC article.
-
Correlation Between Gamma-Glutamyl Transferase Activity and Glutathione Levels in Molecular Subgroups of Breast Cancer.Eur J Breast Health. 2019 Dec 5;16(1):72-76. doi: 10.5152/ejbh.2019.5147. eCollection 2020 Jan. Eur J Breast Health. 2019. PMID: 31912018 Free PMC article.
-
Preoperative D-dimer and Gamma-Glutamyltranspeptidase Predict Major Complications and Survival in Colorectal Liver Metastases Patients After Resection.Transl Oncol. 2019 Jul;12(7):996-1004. doi: 10.1016/j.tranon.2019.04.011. Epub 2019 May 21. Transl Oncol. 2019. PMID: 31125760 Free PMC article.
-
Glutathione: Antioxidant Properties Dedicated to Nanotechnologies.Antioxidants (Basel). 2018 Apr 27;7(5):62. doi: 10.3390/antiox7050062. Antioxidants (Basel). 2018. PMID: 29702624 Free PMC article. Review.
References
-
- Walker BL, Tiong JW, Jefferies WA. Iron metabolism in mammalian cells. Int Rev Cytol. 2001;211:241–278. - PubMed
-
- Weinberg ED. Cellular iron metabolism in health and disease. Drug Metab Rev. 1990;22:531–579. - PubMed
-
- Nunez MT, Gaete V, Watkins JA, Glass J. Mobilization of iron from endocytic vesicles. The effects of acidification and reduction. J Biol Chem. 1990;265:6688–92. - PubMed
-
- Sipe DM, Murphy RF. Binding to cellular receptors results in increased iron release from transferrin at mildly acidic pH. J Biol Chem. 1991;266:8002–8007. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

