Molecular dissection of the inward rectifier potassium current (IK1) in rabbit cardiomyocytes: evidence for heteromeric co-assembly of Kir2.1 and Kir2.2
- PMID: 12794173
- PMCID: PMC2343053
- DOI: 10.1113/jphysiol.2002.036400
Molecular dissection of the inward rectifier potassium current (IK1) in rabbit cardiomyocytes: evidence for heteromeric co-assembly of Kir2.1 and Kir2.2
Abstract
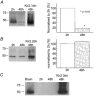
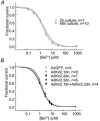
Cardiac inward rectifier K+ currents (IK1) play an important role in maintaining resting membrane potential and contribute to late phase repolarization. Members of the Kir2.x channel family appear to encode for IK1. The purpose of this study was to determine the molecular composition of cardiac IK1 in rabbit ventricle. Western blots revealed that Kir2.1 and Kir2.2, but not Kir2.3, are expressed in rabbit ventricle. Culturing rabbit myocytes resulted in an approximately 50% reduction of IK1 density after 48 or 72 h in culture which was associated with an 80% reduction in Kir2.1, but no change in Kir2.2, protein expression. Dominant-negative (DN) constructs of Kir2.1, Kir2.2 and Kir2.3 were generated and tested in tsA201 cells. Adenovirus-mediated over-expression of Kir2.1dn, Kir2.2dn or Kir2.1dn plus Kir2.2dn in cultured rabbit ventricular myocytes reduced IK1 density equally by 70% 72 h post-infection, while AdKir2.3dn had no effect, compared to green fluorescent protein (GFP)-infected myocytes. Previous studies indicate that the [Ba2+] required for half-maximum block (IC50) differs significantly between Kir2.1, Kir2.2 and Kir2.3 channels. The dependence of IK1 on [Ba2+] revealed a single binding isotherm which did not change with time in culture. The IC50 for block of IK1 was also unaffected by expression of the different DN genes after 72 h in culture. Taken together, these results demonstrate functional expression of Kir2.1 and Kir2.2 in rabbit ventricular myocytes and suggest that macroscopic IK1 is predominantly composed of Kir2.1 and Kir2.2 heterotetramers.
Figures

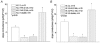

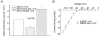

References
-
- Barry DM, Trimmer JS, Merlie JP, Nerbonne JM. Differential expression of voltage-gated K+ channel subunits in adult rat heart. Relation to functional K+ channels. Circ Res. 1995;77:361–369. - PubMed
-
- Brahmajothi MV, Morales MJ, Liu S, Rasmusson RL, Campbell DL, Strauss HC. In situ hybridization reveals extensive diversity of K+ channel mRNA in isolated ferret cardiac myocytes. Circ Res. 1996;78:1083–1089. - PubMed
-
- Burnashev NA, Zilberter YI. Two types of single inward rectifying potassium channels in rat myocardial cells. Gen Physiol Biophys. 1986;5:495–504. - PubMed
-
- Cho HC, Tsushima RG, Nguyen TT, Guy HR, Backx PH. Two critical cysteine residues implicated in disulfide bond formation and proper folding of Kir2. 1. Biochemistry. 2000;39:4649–4657. - PubMed
-
- Christe G. Localization of K(+) channels in the tubules of cardiomyocytes as suggested by the parallel decay of membrane capacitance, IK(1) and IK(ATP) during culture and by delayed IK(1) response to barium. J Mol Cell Cardiol. 1999;31:2207–2213. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

