Interstitial cells: involvement in rhythmicity and neural control of gut smooth muscle
- PMID: 12794179
- PMCID: PMC2343044
- DOI: 10.1113/jphysiol.2003.043299
Interstitial cells: involvement in rhythmicity and neural control of gut smooth muscle
Abstract
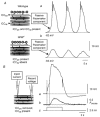
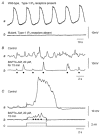
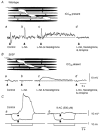
Many smooth muscles display spontaneous electrical and mechanical activity, which persists in the absence of any stimulation. In the past this has been attributed largely to the properties of the smooth muscle cells. Now it appears that in several organs, particularly in the gastrointestinal tract, activity in smooth muscles arises from a separate group of cells, known as interstitial cells of Cajal (ICC), which are distributed amongst the smooth muscle cells. Thus in the gastrointestinal tract, a network of interstitial cells, usually located near the myenteric plexus, generates pacemaker potentials that are conducted passively into the adjacent muscle layers where they produce rhythmical membrane potential changes. The mechanical activity of most smooth muscle cells, can be altered by autonomic, or enteric, nerves innervating them. Previously it was thought that neuroeffector transmission occurred simply because neurally released transmitters acted on smooth muscle cells. However, in several, but not all, regions of the gastrointestinal tract, it appears that nerve terminals, rather than communicating directly with smooth muscle cells, preferentially form synapses with ICC and these relay information to neighbouring smooth muscle cells. Thus a set of ICC, which are distributed amongst the smooth muscle cells of the gut, are the targets of transmitters released by intrinsic enteric excitatory and inhibitory nerve terminals: in some regions of the gastrointestinal tract, the same set of ICC also augment the waves of depolarisation generated by pacemaker ICC. Similarly in the urethra, ICC, distributed amongst the smooth muscle cells, generate rhythmic activity and also appear to be the targets of autonomic nerve terminals.
Figures

References
-
- Cousins HM, Edwards FR, Hirst GD. Neuronally released and applied acetylcholine on the longitudinal muscle of the guinea-pig ileum. Neuroscience. 1995;65:193–207. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

