Transient and sustained types of long-term potentiation in the CA1 area of the rat hippocampus
- PMID: 12794181
- PMCID: PMC2343043
- DOI: 10.1113/jphysiol.2003.044214
Transient and sustained types of long-term potentiation in the CA1 area of the rat hippocampus
Abstract
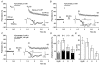
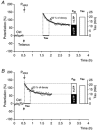
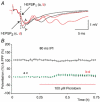
Synaptic potentiation induced by high frequency stimulation was investigated by recording field excitatory postsynaptic potentials (f-EPSPs) in rat hippocampal slices. Potentiation consisted of a transient period of decaying f-EPSPs (short-term potentiation, STP) that led to a plateau of continuously potentiated f-EPSPs (long-term potentiation, LTP). Here we show that a previously unknown type of transient, use-dependent, long-lasting potentiation (t-LTP) can account for STP. t-LTP could be stored for more than 6 h and its decay was caused by synaptic activation. Both the expression and the decay of t-LTP were input specific. t-LTP was induced differently from conventional LTP in that the amplitude of t-LTP was dependent upon the stimulation frequency, whereas the magnitude of LTP was dependent on the number of stimuli in the induction train. The decay of t-LTP could not be prevented by the blockage of glutamate receptors, but was prevented by the blockage of stimulus-evoked neurotransmitter release, suggesting that t-LTP is expressed presynaptically. Paired-pulse stimulation experiments showed that the decay of t-LTP was mediated by a decrease in the probability of neurotransmitter release. The decline of t-LTP could be prolonged by the activation of NMDA receptors. Hence, both single and paired-pulse stimuli prolonged the decline of the t-LTP. This decline could be prevented by high frequency burst stimulation (200 Hz). We conclude that t-LTP allows dynamic modulation of synaptic transmission by providing not only spatial association but also temporal convergence between synaptic inputs. Therefore, t-LTP might be a substrate for the encoding of synaptic memory.
Figures




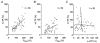






Similar articles
-
N-methyl-D-aspartate receptor-dependent long-term potentiation in CA1 region affects synaptic expression of glutamate receptor subunits and associated proteins in the whole hippocampus.Neuroscience. 2006 Sep 1;141(3):1399-413. doi: 10.1016/j.neuroscience.2006.04.070. Epub 2006 Jun 12. Neuroscience. 2006. PMID: 16766131
-
Plasticity of synaptic GluN receptors is required for the Src-dependent induction of long-term potentiation at CA3-CA1 synapses.Hippocampus. 2011 Oct;21(10):1053-61. doi: 10.1002/hipo.20818. Epub 2010 Jun 2. Hippocampus. 2011. PMID: 20865743
-
Contribution of NMDA receptor channels to the expression of LTP in the hippocampal dentate gyrus.Hippocampus. 2002;12(5):680-8. doi: 10.1002/hipo.10104. Hippocampus. 2002. PMID: 12440582
-
A peculiar form of potentiation in mossy fiber synapses.Epilepsy Res Suppl. 1992;7:151-7. Epilepsy Res Suppl. 1992. PMID: 1334660 Review.
-
[Long-term potentiation of the NMDA-dependent component of the EPSP in the hippocampus].Usp Fiziol Nauk. 1998 Oct-Dec;29(4):6-23. Usp Fiziol Nauk. 1998. PMID: 9883495 Review. Russian.
Cited by
-
Role of reuniens nucleus projections to the medial prefrontal cortex and to the hippocampal pyramidal CA1 area in associative learning.PLoS One. 2011;6(8):e23538. doi: 10.1371/journal.pone.0023538. Epub 2011 Aug 15. PLoS One. 2011. PMID: 21858159 Free PMC article.
-
Side-by-side comparison of the effects of Gq- and Gi-DREADD-mediated astrocyte modulation on intracellular calcium dynamics and synaptic plasticity in the hippocampal CA1.Mol Brain. 2021 Sep 20;14(1):144. doi: 10.1186/s13041-021-00856-w. Mol Brain. 2021. PMID: 34544455 Free PMC article.
-
Two sides to long-term potentiation: a view towards reconciliation.Philos Trans R Soc Lond B Biol Sci. 2013 Dec 2;369(1633):20130154. doi: 10.1098/rstb.2013.0154. Print 2014 Jan 5. Philos Trans R Soc Lond B Biol Sci. 2013. PMID: 24298155 Free PMC article. Review.
-
Different patterns of electrical activity lead to long-term potentiation by activating different intracellular pathways.J Neurosci. 2015 Jan 14;35(2):621-33. doi: 10.1523/JNEUROSCI.2193-14.2015. J Neurosci. 2015. PMID: 25589756 Free PMC article.
-
Multiple roles of GluN2B-containing NMDA receptors in synaptic plasticity in juvenile hippocampus.Neuropharmacology. 2017 Jan;112(Pt A):76-83. doi: 10.1016/j.neuropharm.2016.08.010. Epub 2016 Aug 12. Neuropharmacology. 2017. PMID: 27523302 Free PMC article.
References
-
- Abel T, Nguyen PV, Barad M, Deuel TA, Kandel ER, Bourtchouladze R. Genetic demonstration of a role for PKA in the late phase of LTP and in hippocampus-based long-term memory. Cell. 1997;88:615–626. - PubMed
-
- Albright TD, Jessell TM, Kandel ER, Posner MI. Neural science: a century of progress and the mysteries that remain. Cell. 2000;100:S1–55. - PubMed
-
- Antonova I, Arancio O, Trillat AC, Wang HG, Zablow L, Udo H, Kandel ER, Hawkins RD. Rapid increase in clusters of presynaptic proteins at onset of long-lasting potentiation. Science. 2001;294:1547–1550. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

