The Notch ligand Delta1 is sequentially cleaved by an ADAM protease and gamma-secretase
- PMID: 12794186
- PMCID: PMC164639
- DOI: 10.1073/pnas.1230693100
The Notch ligand Delta1 is sequentially cleaved by an ADAM protease and gamma-secretase
Abstract
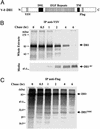
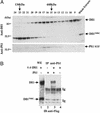
Notch signaling is involved in numerous cell fate decisions in invertebrates and vertebrates. The Notch receptor is a type I transmembrane (TM) protein that undergoes two proteolytic steps after ligand binding, first by an ADAM (a distintegrin and metalloprotease) in the extracellular region, followed by gamma-secretase-mediated cleavage inside the TM domain. We demonstrate here that the murine ligand Delta1 (Dll1) undergoes the same sequence of cleavages, in an apparently signal-independent manner. Identification of the ADAM-mediated shedding site localized 10 aa N-terminal to the TM domain has enabled us to generate a noncleavable mutant. Kuzbanian/ADAM10 is involved in this processing event, but other proteases can probably substitute for it. We then show that Dll1 is part of a high-molecular-weight complex containing presenilin1 and undergoes further cleavage by a gamma-secretase-like activity, therefore releasing the intracellular domain that localizes in part to the nucleus. Using the shedding-resistant mutant, we demonstrate that this gamma-secretase cleavage depends on prior ectodomain shedding. Therefore Dll1 is a substrate for regulated intramembrane proteolysis, and its intracellular region possibly fulfills a specific function in the nucleus.
Figures



References
-
- Artavanis-Tsakonas, S., Rand, M. D. & Lake, R. J. (1999) Science 284, 770–776. - PubMed
-
- Blaumueller, C. M., Qi, H. L., Zagouras, P. & Artavanis-Tsakonas, S. (1997) Cell 90, 281–291. - PubMed
-
- Fleming, R. J., Purcell, K. & Artavanis-Tsakonas, S. (1997) Trends Cell Biol. 7, 437–441. - PubMed
-
- Lendahl, U. (1998) BioEssays 20, 103–107. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

