Simplified synthetic TMC-95A/B analogues retain the potency of proteasome inhibitory activity
- PMID: 12794861
- PMCID: PMC2556569
- DOI: 10.1002/cbic.200300560
Simplified synthetic TMC-95A/B analogues retain the potency of proteasome inhibitory activity
Abstract
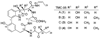
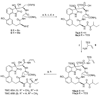
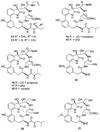
The proteasome regulates diverse intracellular processes, including cell-cycle progression, antigen presentation, and inflammatory response. Selective inhibitors of the proteasome have great therapeutic potential for the treatment of cancer and inflammatory disorders. Natural cyclic peptides TMC-95A and B represent a new class of noncovalent, selective proteasome inhibitors. To explore the structure-activity relationship of this class of proteasome inhibitors, a series of TMC-95A/B analogues were prepared and analyzed. We found that the unique enamide functionality at the C8 position of TMC-95s can be replaced with a simple allylamide. The asymmetric center at C36 that distinguishes TMC-95A from TMC-95B but which necessitates a complicated separation of the two compounds can be eliminated. Therefore, these findings could lead to the development of more accessible simple analogues as potential therapeutic agents.
Figures
Similar articles
-
Total synthesis of TMC-95A and -B via a new reaction leading to Z-enamides. Some preliminary findings as to SAR.J Am Chem Soc. 2004 May 26;126(20):6347-55. doi: 10.1021/ja049821k. J Am Chem Soc. 2004. PMID: 15149232 Free PMC article.
-
The total synthesis of proteasome inhibitors TMC-95A and TMC-95B: discovery of a new method to generate cis-propenyl amides.Angew Chem Int Ed Engl. 2002 Feb 1;41(3):512-5. doi: 10.1002/1521-3773(20020201)41:3<512::aid-anie512>3.0.co;2-r. Angew Chem Int Ed Engl. 2002. PMID: 12491396 No abstract available.
-
Crystal structure of the 20 S proteasome:TMC-95A complex: a non-covalent proteasome inhibitor.J Mol Biol. 2001 Aug 17;311(3):543-8. doi: 10.1006/jmbi.2001.4869. J Mol Biol. 2001. PMID: 11493007
-
Recent advances in the identification and development of 20S proteasome inhibitors.Mini Rev Med Chem. 2002 Jun;2(3):247-59. doi: 10.2174/1389557023406142. Mini Rev Med Chem. 2002. PMID: 12370066 Review.
-
The ubiquitin-proteasome pathway and proteasome inhibitors.Med Res Rev. 2001 Jul;21(4):245-73. doi: 10.1002/med.1009. Med Res Rev. 2001. PMID: 11410931 Free PMC article. Review.
Cited by
-
Natural product scaffolds as inspiration for the design and synthesis of 20S human proteasome inhibitors.RSC Chem Biol. 2020 Dec 1;1(5):305-332. doi: 10.1039/d0cb00111b. Epub 2020 Sep 16. RSC Chem Biol. 2020. PMID: 33791679 Free PMC article.
-
Total synthesis of TMC-95A and -B via a new reaction leading to Z-enamides. Some preliminary findings as to SAR.J Am Chem Soc. 2004 May 26;126(20):6347-55. doi: 10.1021/ja049821k. J Am Chem Soc. 2004. PMID: 15149232 Free PMC article.
-
A concise, total synthesis of the TMC-95A/B proteasome inhibitors.Proc Natl Acad Sci U S A. 2004 Aug 17;101(33):11949-54. doi: 10.1073/pnas.0308432101. Epub 2004 May 26. Proc Natl Acad Sci U S A. 2004. PMID: 15163792 Free PMC article.
-
Access to 3,3-disubstituted oxindoles via microwave-assisted Cannizzaro and aldol reactions of formaldehyde with isatins and their imines.RSC Adv. 2021 May 11;11(28):17320-17323. doi: 10.1039/d1ra02150h. eCollection 2021 May 6. RSC Adv. 2021. PMID: 35479673 Free PMC article.
-
Inhibition of human and yeast 20S proteasome by analogues of trypsin inhibitor SFTI-1.PLoS One. 2014 Feb 25;9(2):e89465. doi: 10.1371/journal.pone.0089465. eCollection 2014. PLoS One. 2014. PMID: 24586798 Free PMC article.
References
-
- Rock KL, Gramm C, Rothstein L, Clark K, Stein R, Dick L, Hwang D, Goldberg AL. Cell. 1994;78:761–771. - PubMed
-
- Craiu A, Gaczynska M, Akopian T, Gramm CF, Fenteany G, Goldberg AL, Rock KL. J. Biol. Chem. 1997;272:13437–13445. - PubMed
-
- Hershko A, Ciechanover A. Annu. Rev. Biochem. 1998;67:425–479. - PubMed
-
- Coux O, Tanaka K, Goldberg AL. Annu. Rev. Biochem. 1996;65:801–847. - PubMed
-
- Baumeister W, Walz J, Zuhl F, Seemuller E. Cell. 1998;92:367–380. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

