Insulin signaling inhibits the 5-HT2C receptor in choroid plexus via MAP kinase
- PMID: 12795815
- PMCID: PMC165579
- DOI: 10.1186/1471-2202-4-10
Insulin signaling inhibits the 5-HT2C receptor in choroid plexus via MAP kinase
Abstract
Background: G protein-coupled receptors (GPCRs) interact with heterotrimeric GTP-binding proteins (G proteins) to modulate acute changes in intracellular messenger levels and ion channel activity. In contrast, long-term changes in cellular growth, proliferation and differentiation are often mediated by tyrosine kinase receptors and certain GPCRs by activation of mitogen-activated protein (MAP) kinases. Complex interactions occur between these signaling pathways, but the specific mechanisms of such regulatory events are not well-understood. In particular it is not clear whether GPCRs are modulated by tyrosine kinase receptor-MAP kinase pathways.
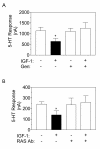
Results: Here we describe tyrosine kinase receptor regulation of a GPCR via MAP kinase. Insulin reduced the activity of the 5-HT2C receptor in choroid plexus cells which was blocked by the MAP kinase kinase (MEK) inhibitor, PD 098059. We demonstrate that the inhibitory effect of insulin and insulin-like growth factor type 1 (IGF-1) on the 5-HT2C receptor is dependent on tyrosine kinase, RAS and MAP kinase. The effect may be receptor-specific: insulin had no effect on another GPCR that shares the same G protein signaling pathway as the 5-HT2C receptor. This effect is also direct: activated MAP kinase mimicked the effect of insulin, and removing a putative MAP kinase site from the 5-HT2C receptor abolished the effect of insulin.
Conclusion: These results show that insulin signaling can inhibit 5-HT2C receptor activity and suggest that MAP kinase may play a direct role in regulating the function of a specific GPCR.
Figures


Similar articles
-
Inhibition of mitogen-activated protein kinase kinase selectively inhibits cell proliferation in human breast cancer cells displaying enhanced insulin-like growth factor I-mediated mitogen-activated protein kinase activation.Cell Growth Differ. 2000 Dec;11(12):655-64. Cell Growth Differ. 2000. PMID: 11149601
-
Role of protein kinase C, PI3-kinase and tyrosine kinase in activation of MAP kinase by glucose and agonists of G-protein coupled receptors in INS-1 cells.Int J Exp Diabetes Res. 2001;2(3):233-44. doi: 10.1155/edr.2001.233. Int J Exp Diabetes Res. 2001. PMID: 12369712 Free PMC article.
-
Tumor necrosis factor alpha-mediated insulin resistance, but not dedifferentiation, is abrogated by MEK1/2 inhibitors in 3T3-L1 adipocytes.Mol Endocrinol. 2000 Oct;14(10):1557-69. doi: 10.1210/mend.14.10.0542. Mol Endocrinol. 2000. PMID: 11043572
-
A short history of the 5-HT2C receptor: from the choroid plexus to depression, obesity and addiction treatment.Psychopharmacology (Berl). 2017 May;234(9-10):1395-1418. doi: 10.1007/s00213-017-4545-5. Epub 2017 Mar 7. Psychopharmacology (Berl). 2017. PMID: 28265714 Review.
-
Composition and function of g protein-coupled receptor signalsomes controlling mitogen-activated protein kinase activity.J Mol Neurosci. 2005;26(2-3):253-64. doi: 10.1385/JMN:26:2-3:253. J Mol Neurosci. 2005. PMID: 16012199 Review.
Cited by
-
Insulin in the brain: there and back again.Pharmacol Ther. 2012 Oct;136(1):82-93. doi: 10.1016/j.pharmthera.2012.07.006. Epub 2012 Jul 17. Pharmacol Ther. 2012. PMID: 22820012 Free PMC article. Review.
-
Release of insulin produced by the choroid plexis is regulated by serotonergic signaling.JCI Insight. 2019 Dec 5;4(23):e131682. doi: 10.1172/jci.insight.131682. JCI Insight. 2019. PMID: 31647782 Free PMC article.
-
Serotonin and the regulation of mammalian energy balance.Front Neurosci. 2013 Mar 27;7:36. doi: 10.3389/fnins.2013.00036. eCollection 2013. Front Neurosci. 2013. PMID: 23543912 Free PMC article.
-
Cerebral insulin, insulin signaling pathway, and brain angiogenesis.Neurol Sci. 2016 Jan;37(1):9-16. doi: 10.1007/s10072-015-2386-8. Epub 2015 Oct 6. Neurol Sci. 2016. PMID: 26442674 Review.
-
Serotonin transporter (SERT) and translocator protein (TSPO) expression in the obese ob/ob mouse.BMC Neurosci. 2011 Feb 7;12:18. doi: 10.1186/1471-2202-12-18. BMC Neurosci. 2011. PMID: 21299850 Free PMC article.
References
-
- Marinissen MJ, Gutkind JS. G-protein-coupled receptors and signaling networks: emerging paradigms. Trends Pharmacol Sci. 2001;22:368–376. - PubMed
-
- Luttrell LM. Activation and targeting of mitogen-activated protein kinases by G-protein-coupled receptors. Can J Physiol Pharmacol. 2002;80:375–382. - PubMed
-
- van Biesen T, Luttrell LM, Hawes BE, Lefkowitz RJ. Mitogenic signaling via G protein-coupled receptors. Endocr Rev. 1996;17:698–714. - PubMed
-
- Lauder JM. Neurotransmitters as growth regulatory signals: role of receptors and second messengers. Trends NeuroSci. 1993;16:233–240. - PubMed
-
- Gerhardt CC, van Heerikhuizen H. Functional characteristics of heterologously expressed 5-HT receptors. Eur J Pharmacol. 1997;334:1–23. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

