Food transport in the C. elegans pharynx
- PMID: 12796460
- PMCID: PMC3951750
- DOI: 10.1242/jeb.00433
Food transport in the C. elegans pharynx
Abstract
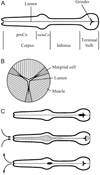
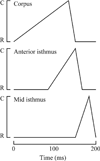
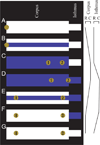
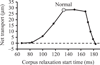
Pumping of the C. elegans pharynx transports food particles (bacteria) posteriorly. We examined muscle motions to determine how this posterior transport is effected. We find that the motions of the middle section of the pharynx, the anterior isthmus, are delayed relative to the anterior section, the corpus. Simulations in which particles are assumed to move at mean fluid velocity when not captured by the walls of the pharyngeal lumen show that delayed isthmus motions do indeed cause net particle transport; however, the amount is much less than in the real pharynx. We propose that the geometry of the pharyngeal lumen forces particles to the center, where they move faster than mean fluid velocity. When this acceleration is incorporated into the simulation, particles are transported efficiently. The transport mechanism we propose explains past observations that the timing of muscle relaxation is important for effective transport. Our model also makes a prediction, which we confirm, that smaller bacteria are better food sources for C. elegans than large ones.
Figures







References
-
- Albertson DG, Thomson JN. The pharynx of Caenorhabditis elegans. Phil. Trans. R. Soc. Lond. B. 1976;275:299–325. - PubMed
-
- Avery L. Motor neuron M3 controls pharyngeal muscle relaxation timing in Caenorhabditis elegans. J. Exp. Biol. 1993b;175:283–297. - PubMed
-
- Avery L, Horvitz HR. Pharyngeal pumping continues after laser killing of the pharyngeal nervous system.of C. elegans. Neuron. 1989;3:473–485. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

