A reversible defect in natural killer T cell function characterizes the progression of premalignant to malignant multiple myeloma
- PMID: 12796469
- PMCID: PMC2193955
- DOI: 10.1084/jem.20021650
A reversible defect in natural killer T cell function characterizes the progression of premalignant to malignant multiple myeloma
Abstract
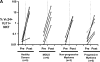
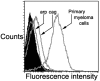
We studied the function of antitumor T and natural killer T (NKT) cells from the blood and tumor bed in 23 patients with premalignant gammopathy, nonprogressive myeloma, or progressive multiple myeloma. We show that antitumor killer T cells can be detected in patients with both progressive or nonprogressive myeloma. V alpha 24+V beta 11+ invariant NKT cells are detectable in the blood and tumor bed of all cohorts. However, freshly isolated NKT cells from both the blood and tumor bed of patients with progressive disease, but not nonprogressive myeloma or premalignant gammopathy, have a marked deficiency of ligand-dependent interferon-gamma production. This functional defect can be overcome in vitro using dendritic cells pulsed with the NKT ligand, alpha-galactosylceramide (alpha-GalCer). Fresh myeloma cells express CD1d, and can be efficiently killed by autologous NKT cells. We hypothesize that presentation of tumor derived glycolipids by myeloma cells leads to NKT dysfunction in vivo. These data demonstrate that clinical progression in patients with monoclonal gammopathies is associated with an acquired but potentially reversible defect in NKT cell function and support the possibility that these innate lymphocytes play a role in controlling the malignant growth of this incurable B cell tumor in patients.
Figures











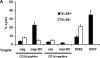
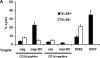
Similar articles
-
Phenotypical and functional alterations during the expansion phase of invariant Valpha14 natural killer T (Valpha14i NKT) cells in mice primed with alpha-galactosylceramide.Immunology. 2005 Sep;116(1):30-7. doi: 10.1111/j.1365-2567.2005.02193.x. Immunology. 2005. PMID: 16108815 Free PMC article.
-
Application of natural killer T cells in antitumor immunotherapy.Crit Rev Immunol. 2007;27(6):511-25. doi: 10.1615/critrevimmunol.v27.i6.20. Crit Rev Immunol. 2007. PMID: 18197797 Review.
-
Prevention of injury-induced suppression of T-cell immunity by the CD1d/NKT cell-specific ligand alpha-galactosylceramide.Shock. 2008 Feb;29(2):269-77. doi: 10.1097/shk.0b013e31811ff60c. Shock. 2008. PMID: 17693934
-
CpG ODN enhance antigen-specific NKT cell activation via plasmacytoid dendritic cells.Eur J Immunol. 2005 Aug;35(8):2347-57. doi: 10.1002/eji.200425721. Eur J Immunol. 2005. PMID: 16025562
-
Innate Valpha14(+) natural killer T cells mature dendritic cells, leading to strong adaptive immunity.Immunol Rev. 2007 Dec;220:183-98. doi: 10.1111/j.1600-065X.2007.00561.x. Immunol Rev. 2007. PMID: 17979847 Review.
Cited by
-
Can we change the disease biology of multiple myeloma?Leuk Res. 2012 Nov;36 Suppl 1(0 1):S3-12. doi: 10.1016/S0145-2126(12)70003-6. Leuk Res. 2012. PMID: 23176722 Free PMC article. Review.
-
Cancer Immunotherapeutic Potential of NKTT320, a Novel, Invariant, Natural Killer T Cell-Activating, Humanized Monoclonal Antibody.Int J Mol Sci. 2020 Jun 17;21(12):4317. doi: 10.3390/ijms21124317. Int J Mol Sci. 2020. PMID: 32560408 Free PMC article.
-
Immunomodulatory effects of lenalidomide and pomalidomide on interaction of tumor and bone marrow accessory cells in multiple myeloma.Blood. 2010 Oct 28;116(17):3227-37. doi: 10.1182/blood-2010-04-279893. Epub 2010 Jul 22. Blood. 2010. PMID: 20651070 Free PMC article.
-
Tissue-Resident Innate Immune Cell-Based Therapy: A Cornerstone of Immunotherapy Strategies for Cancer Treatment.Front Cell Dev Biol. 2022 May 26;10:907572. doi: 10.3389/fcell.2022.907572. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35757002 Free PMC article. Review.
-
iNKT cell cytotoxic responses control T-lymphoma growth in vitro and in vivo.Cancer Immunol Res. 2014 Jan;2(1):59-69. doi: 10.1158/2326-6066.CIR-13-0104. Cancer Immunol Res. 2014. PMID: 24563871 Free PMC article.
References
-
- Bernards, R., and R.A. Weinberg. 2002. A progression puzzle. Nature. 418:823. - PubMed
-
- Rawstron, A.C., M.J. Green, A. Kuzmicki, B. Kennedy, J.A. Fenton, P.A. Evans, S.J. O'Connor, S.J. Richards, G.J. Morgan, A.S. Jack, and P. Hillmen. 2002. Monoclonal B lymphocytes with the characteristics of “indolent” chronic lymphocytic leukemia are present in 3.5% of adults with normal blood counts. Blood. 100:635–639. - PubMed
-
- Kyle, R.A., and S.V. Rajkumar. 1999. Monoclonal gammopathies of undetermined significance. Hematol. Oncol. Clin. North Am. 13:1181–1202. - PubMed
-
- Tricot, G. 2000. Multiple myeloma and related plasma cell disorders. Hematology: Principles and Practice. R. Hoffman, editor. Churchill Livingstone, New York. 1398–1415.
-
- Fonseca, R., R.J. Bailey, G.J. Ahmann, S.V. Rajkumar, J.D. Hoyer, J.A. Lust, R.A. Kyle, M.A. Gertz, P.R. Greipp, and G.W. Dewald. 2002. Genomic abnormalities in monoclonal gammopathy of undetermined significance. Blood. 100:1417–1424. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

