Regulation of leading edge microtubule and actin dynamics downstream of Rac1
- PMID: 12796474
- PMCID: PMC2172968
- DOI: 10.1083/jcb.200303082
Regulation of leading edge microtubule and actin dynamics downstream of Rac1
Abstract
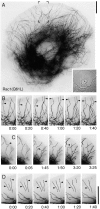
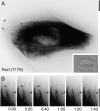
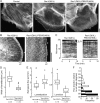
Actin in migrating cells is regulated by Rho GTPases. However, Rho proteins might also affect microtubules (MTs). Here, we used time-lapse microscopy of PtK1 cells to examine MT regulation downstream of Rac1. In these cells, "pioneer" MTs growing into leading-edge protrusions exhibited a decreased catastrophe frequency and an increased time in growth as compared with MTs further from the leading edge. Constitutively active Rac1(Q61L) promoted pioneer behavior in most MTs, whereas dominant-negative Rac1(T17N) eliminated pioneer MTs, indicating that Rac1 is a regulator of MT dynamics in vivo. Rac1(Q61L) also enhanced MT turnover through stimulation of MT retrograde flow and breakage. Inhibition of p21-activated kinases (Paks), downstream effectors of Rac1, inhibited Rac1(Q61L)-induced MT growth and retrograde flow. In addition, Rac1(Q61L) promoted lamellipodial actin polymerization and Pak-dependent retrograde flow. Together, these results indicate coordinated regulation of the two cytoskeletal systems in the leading edge of migrating cells.
Figures


References
-
- Adams, M.C., W.C. Salmon, S.L. Gupton, C.S. Cohan, T. Wittmann, N. Prigozhina, and C.M. Waterman-Storer. 2003. A high-speed multispectral spinning-disk confocal microscope system for fluorescent speckle microscopy of living cells. Methods. 29:29–41. - PubMed
-
- Bokoch, G.M. 2003. Biology of the p21-activated kinases. Annu. Rev. Biochem. 72:743–781. - PubMed
-
- Daub, H., K. Gevaert, J. Vandekerckhove, A. Sobel, and A. Hall. 2001. Rac/Cdc42 and p65PAK regulate the microtubule-destabilizing protein stathmin through phosphorylation at serine 16. J. Biol. Chem. 276:1677–1680. - PubMed
-
- Desai, A., and T.J. Mitchison. 1997. Microtubule polymerization dynamics. Annu. Rev. Cell Dev. Biol. 13:83–117. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

