Requirement of transcription factor NFAT in developing atrial myocardium
- PMID: 12796475
- PMCID: PMC2172977
- DOI: 10.1083/jcb.200301058
Requirement of transcription factor NFAT in developing atrial myocardium
Abstract
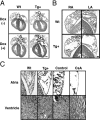
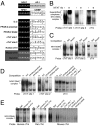
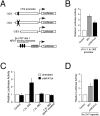
Nuclear factor of activated T cell (NFAT) is a ubiquitous regulator involved in multiple biological processes. Here, we demonstrate that NFAT is temporally required in the developing atrial myocardium between embryonic day 14 and P0 (birth). Inhibition of NFAT activity by conditional expression of dominant-negative NFAT causes thinning of the atrial myocardium. The thin myocardium exhibits severe sarcomere disorganization and reduced expression of cardiac troponin-I (cTnI) and cardiac troponin-T (cTnT). Promoter analysis indicates that NFAT binds to and regulates transcription of the cTnI and the cTnT genes. Thus, regulation of cytoskeletal protein gene expression by NFAT may be important for the structural architecture of the developing atrial myocardium.
Figures









References
-
- Agarwal, S., O. Avni, and A. Rao. 2000. Cell-type-restricted binding of the transcription factor NFAT to a distal IL-4 enhancer in vivo. Immunity. 12:643–652. - PubMed
-
- Aramburu, J., F. Garcia-Cozar, A. Raghavan, H. Okamura, A. Rao, and P.G. Hogan. 1998. Selective inhibition of NFAT activation by a peptide spanning the calcineurin targeting site of NFAT. Mol. Cell. 1:627–637. - PubMed
-
- Ausoni, S., M. Campione, A. Picard, P. Moretti, M. Vitadello, C. De Nardi, and S. Schiaffino. 1994. Structure and regulation of the mouse cardiac troponin I gene. J. Biol. Chem. 269:339–346. - PubMed
-
- Barton, P.J., M.E. Cullen, P.J. Townsend, N.J. Brand, M.J. Mullen, D.A. Norman, P.K. Bhavsar, and M.H. Yacoub. 1999. Close physical linkage of human troponin genes: organization, sequence and expression of the locus encoding cardiac troponin I and slow skeletal troponin T. Genomics. 57:102–109. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

