Brain-derived neurotrophic factor regulates energy balance downstream of melanocortin-4 receptor
- PMID: 12796784
- PMCID: PMC2710100
- DOI: 10.1038/nn1073
Brain-derived neurotrophic factor regulates energy balance downstream of melanocortin-4 receptor
Abstract
The melanocortin-4 receptor (MC4R) is critically involved in regulating energy balance, and obesity has been observed in mice with mutations in the gene for brain-derived neurotrophic factor (BDNF). Here we report that BDNF is expressed at high levels in the ventromedial hypothalamus (VMH) where its expression is regulated by nutritional state and by MC4R signaling. In addition, similar to MC4R mutants, mouse mutants that expresses the BDNF receptor TrkB at a quarter of the normal amount showed hyperphagia and excessive weight gain on higher-fat diets. Furthermore, BDNF infusion into the brain suppressed the hyperphagia and excessive weight gain observed on higher-fat diets in mice with deficient MC4R signaling. These results show that MC4R signaling controls BDNF expression in the VMH and support the hypothesis that BDNF is an important effector through which MC4R signaling controls energy balance.
Figures

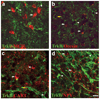
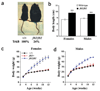


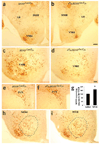
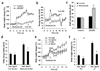
Comment in
-
The skinny on neurotrophins.Nat Neurosci. 2003 Jul;6(7):655-6. doi: 10.1038/nn0703-655. Nat Neurosci. 2003. PMID: 12830151 No abstract available.
References
-
- Rios M, et al. Conditional deletion of brain-derived neurotrophic factor in the postnatal brain leads to obesity and hyperactivity. Mol. Endocrinol. 2001;15:1748–1757. - PubMed
-
- Ono M, et al. Brain-derived neurotrophic factor reduces blood glucose level in obese diabetic mice but not in normal mice. Biochem. Biophys. Res. Commun. 1997;238:633–637. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

