Brief calorie restriction increases Akt2 phosphorylation in insulin-stimulated rat skeletal muscle
- PMID: 12799317
- PMCID: PMC2748752
- DOI: 10.1152/ajpendo.00224.2003
Brief calorie restriction increases Akt2 phosphorylation in insulin-stimulated rat skeletal muscle
Abstract
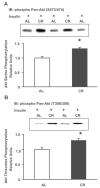
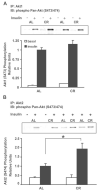
Skeletal muscle insulin sensitivity improves with short-term reduction in calorie intake. The goal of this study was to evaluate changes in the abundance and phosphorylation of Akt1 and Akt2 as potential mechanisms for enhanced insulin action after 20 days of moderate calorie restriction [CR; 60% of ad libitum (AL) intake] in rat skeletal muscle. We also assessed changes in the abundance of SH2 domain-containing inositol phosphatase (SHIP2), a negative regulator of insulin signaling. Fisher 344 x Brown Norway rats were assigned to an AL control group or a CR treatment group for 20 days. Epitrochlearis muscles were dissected and incubated with or without insulin (500 microU/ml). Total Akt serine and threonine phosphorylation was significantly increased by 32 (P < 0.01) and 30% (P < 0.005) in insulin-stimulated muscles from CR vs. AL. Despite an increase in total Akt phosphorylation, there was no difference in Akt1 serine or Akt1 threonine phosphorylation between CR and AL insulin-treated muscles. However, there was a 30% decrease (P < 0.05) in Akt1 abundance for CR vs. AL. In contrast, there was no change in Akt2 protein abundance, and there was a 94% increase (P < 0.05) in Akt2 serine phosphorylation and an increase of 75% (P < 0.05) in Akt2 threonine phosphorylation of insulin-stimulated CR muscles compared with AL. There was no diet effect on SHIP2 abundance in skeletal muscle. These results suggest that, with brief CR, enhanced Akt2 phosphorylation may play a role in increasing insulin sensitivity in rat skeletal muscles.
Figures



Similar articles
-
Akt2 is essential for the full effect of calorie restriction on insulin-stimulated glucose uptake in skeletal muscle.Diabetes. 2005 May;54(5):1349-56. doi: 10.2337/diabetes.54.5.1349. Diabetes. 2005. PMID: 15855319
-
Preventing the calorie restriction-induced increase in insulin-stimulated Akt2 phosphorylation eliminates calorie restriction's effect on glucose uptake in skeletal muscle.Biochim Biophys Acta. 2012 Nov;1822(11):1735-40. doi: 10.1016/j.bbadis.2012.07.012. Epub 2012 Jul 27. Biochim Biophys Acta. 2012. PMID: 22846604 Free PMC article.
-
Heterogeneous effects of calorie restriction on in vivo glucose uptake and insulin signaling of individual rat skeletal muscles.PLoS One. 2013 Jun 3;8(6):e65118. doi: 10.1371/journal.pone.0065118. Print 2014. PLoS One. 2013. PMID: 23755179 Free PMC article.
-
Calorie restriction leads to greater Akt2 activity and glucose uptake by insulin-stimulated skeletal muscle from old rats.Am J Physiol Regul Integr Comp Physiol. 2016 Mar 1;310(5):R449-58. doi: 10.1152/ajpregu.00449.2015. Epub 2016 Jan 6. Am J Physiol Regul Integr Comp Physiol. 2016. PMID: 26739650 Free PMC article.
-
In vitro simulation of calorie restriction-induced decline in glucose and insulin leads to increased insulin-stimulated glucose transport in rat skeletal muscle.Am J Physiol Endocrinol Metab. 2007 Dec;293(6):E1782-8. doi: 10.1152/ajpendo.00531.2007. Epub 2007 Oct 9. Am J Physiol Endocrinol Metab. 2007. PMID: 17925453
Cited by
-
Quantitative Phosphoproteomic Analysis Reveals the Regulatory Networks of Elovl6 on Lipid and Glucose Metabolism in Zebrafish.Int J Mol Sci. 2020 Apr 19;21(8):2860. doi: 10.3390/ijms21082860. Int J Mol Sci. 2020. PMID: 32325903 Free PMC article.
-
Improved cardiac metabolism and activation of the RISK pathway contributes to improved post-ischemic recovery in calorie restricted mice.J Mol Med (Berl). 2011 Mar;89(3):291-302. doi: 10.1007/s00109-010-0703-5. Epub 2010 Dec 8. J Mol Med (Berl). 2011. PMID: 21140129
-
Improved insulin sensitivity by calorie restriction is associated with reduction of ERK and p70S6K activities in the liver of obese Zucker rats.J Endocrinol. 2009 Dec;203(3):337-47. doi: 10.1677/JOE-09-0181. Epub 2009 Oct 2. J Endocrinol. 2009. PMID: 19801385 Free PMC article.
-
Improved insulin sensitivity with calorie restriction does not require reduced JNK1/2, p38, or ERK1/2 phosphorylation in skeletal muscle of 9-month-old rats.Am J Physiol Regul Integr Comp Physiol. 2012 Jan 1;302(1):R126-36. doi: 10.1152/ajpregu.00372.2011. Epub 2011 Oct 19. Am J Physiol Regul Integr Comp Physiol. 2012. PMID: 22012698 Free PMC article.
-
Tissue-specific responses of IGF-1/insulin and mTOR signaling in calorie restricted rats.PLoS One. 2012;7(6):e38835. doi: 10.1371/journal.pone.0038835. Epub 2012 Jun 6. PLoS One. 2012. PMID: 22701721 Free PMC article.
References
-
- Andjelkovic M, Alessi DR, Meier R, Fernandez A, Lamb NJ, Frech M, Cron P, Cohen P, Lucocq JM, Hemmings BA. Role of translocation in the activation and function of protein kinase B. J Biol Chem. 1997;272:31515–31524. - PubMed
-
- Arciero PJ, Vukovich MD, Holloszy JO, Racette SB, Kohrt WM. Comparison of short-term diet and exercise on insulin action in individuals with abnormal glucose tolerance. J Appl Physiol. 1999;86:1930–1935. - PubMed
-
- Balage M, Grizard J, Sornet C, Simon J, Dardevet D, Manin M. Insulin binding and receptor tyrosine kinase activity in rat liver and skeletal muscle: effect of starvation. Metabolism. 1990;39:366–373. - PubMed
-
- Blero D, De Smedt F, Pesesse X, Paternotte N, Moreau C, Payrastre B, Erneux C. The SH2 domain containing inositol 5-phosphatase SHIP2 controls phosphatidylinositol 3,4,5-trisphosphate levels in CHO-IR cells stimulated by insulin. Biochem Biophys Res Commun. 2001;282:839–843. - PubMed
-
- Brodbeck D, Cron P, Hemmings BA. A human protein kinase Bγ with regulatory phosphorylation sites in the activation loop and in the C-terminal hydrophobic domain. J Biol Chem. 1999;274:9133–9136. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

