Identification of novel co-repressor molecules for Interferon Regulatory Factor-2
- PMID: 12799427
- PMCID: PMC162335
- DOI: 10.1093/nar/gkg431
Identification of novel co-repressor molecules for Interferon Regulatory Factor-2
Abstract
We have identified two novel proteins that interact specifically with the C-terminal repression domain of Interferon Regulatory Factor-2 (IRF-2). These proteins, which we term IRF-2 binding proteins 1 and 2 (IRF-2BP1 and IRF-2BP2, the latter having two splicing isoforms, A and B), are nuclear proteins, and have the properties of IRF-2-dependent transcriptional co-repressors that can inhibit both enhancer-activated and basal transcription in a manner that is not dependent upon histone deacetylation. IRF-2BP1 and IRF-2BP2A/B contain an N-terminal zinc finger and a C-terminal RING finger domain of the C3HC4 subclass, but show no homology to other known transcriptional regulators; they therefore define a new family of co- repressor proteins. An alternatively spliced form of IRF-2 that lacks two amino acids (valines 177 and 178) in the central portion of the protein (IRF-2[S]) cannot bind to these co-repressors and cannot mediate repression despite having the same C- terminal repression domain as IRF-2, suggesting that the relative conformation of the DNA binding domain and the C-terminal region of IRF-2 is crucial for transcriptional repression.
Figures




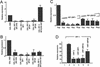

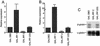
Similar articles
-
Interferon regulatory factor 2 binding protein 2 is a new NFAT1 partner and represses its transcriptional activity.Mol Cell Biol. 2011 Jul;31(14):2889-901. doi: 10.1128/MCB.00974-10. Epub 2011 May 16. Mol Cell Biol. 2011. PMID: 21576369 Free PMC article.
-
Cloning of an interferon regulatory factor 2 isoform with different regulatory ability.Nucleic Acids Res. 2000 Nov 1;28(21):4219-24. doi: 10.1093/nar/28.21.4219. Nucleic Acids Res. 2000. PMID: 11058120 Free PMC article.
-
The BCL-6 POZ domain and other POZ domains interact with the co-repressors N-CoR and SMRT.Oncogene. 1998 Nov 12;17(19):2473-84. doi: 10.1038/sj.onc.1202197. Oncogene. 1998. PMID: 9824158
-
Modulation of thyroid hormone receptor silencing function by co-repressors and a synergizing transcription factor.Biochem Soc Trans. 2000;28(4):386-9. Biochem Soc Trans. 2000. PMID: 10961925 Review.
-
Zinc finger protein ZNF202 structure and function in transcriptional control of HDL metabolism.Curr Opin Lipidol. 2004 Apr;15(2):199-208. doi: 10.1097/00041433-200404000-00013. Curr Opin Lipidol. 2004. PMID: 15017363 Review.
Cited by
-
IRF2BP2 3'UTR Polymorphism Increases Coronary Artery Calcification in Men.Front Cardiovasc Med. 2021 Oct 25;8:687645. doi: 10.3389/fcvm.2021.687645. eCollection 2021. Front Cardiovasc Med. 2021. PMID: 34760935 Free PMC article.
-
Identification of novel NPRAP/δ-catenin-interacting proteins and the direct association of NPRAP with dynamin 2.PLoS One. 2011;6(10):e25379. doi: 10.1371/journal.pone.0025379. Epub 2011 Oct 14. PLoS One. 2011. PMID: 22022388 Free PMC article.
-
Identification and Functional Characterization of a Novel Androgen Receptor Coregulator, EAP1.J Endocr Soc. 2021 Sep 13;5(11):bvab150. doi: 10.1210/jendso/bvab150. eCollection 2021 Nov 1. J Endocr Soc. 2021. PMID: 34585037 Free PMC article.
-
Loss of IRF2BP2 in Microglia Increases Inflammation and Functional Deficits after Focal Ischemic Brain Injury.Front Cell Neurosci. 2017 Jul 19;11:201. doi: 10.3389/fncel.2017.00201. eCollection 2017. Front Cell Neurosci. 2017. PMID: 28769762 Free PMC article.
-
Identification of coilin interactors reveals coordinated control of Cajal body number and structure.J Cell Biol. 2025 Feb 3;224(2):e202305081. doi: 10.1083/jcb.202305081. Epub 2024 Nov 27. J Cell Biol. 2025. PMID: 39602297 Free PMC article.
References
-
- Taniguchi T., Ogasawara,K., Takaoka,A. and Tanaka,N. (2001) IRF family of transcription factors as regulators of host defense. Annu. Rev. Immunol., 19, 623–655. - PubMed
-
- Harada H., Fujita,T., Miyamoto,M., Kimura,Y., Maruyama,M., Furia,A., Miyata,T. and Taniguchi,T. (1989) Structurally similar but functionally distinct factors, IRF-1 and IRF-2, bind to the same regulatory elements of IFN and IFN-inducible genes. Cell, 58, 729–739. - PubMed
-
- Hida S., Ogasawara,K., Sato,K., Abe,M., Takayanagi,H., Yokochi,T., Sato,T., Hirose,S., Shirai,T., Taki,S. and Taniguchi,T. (2000) CD8(+) T cell-mediated skin disease in mice lacking IRF-2, the transcriptional attenuator of interferon-alpha/beta signaling. Immunity, 13, 643–655. - PubMed
-
- Harada H., Kitagawa,M., Tanaka,N., Yamamoto,H., Harada,K., Ishihara,M. and Taniguchi,T. (1993) Anti-oncogenic and oncogenic potentials of interferon regulatory factors-1 and -2. Science, 259, 971–974. - PubMed
-
- Nguyen H., Mustafa,A., Hiscott,J. and Lin,R. (1995) Transcription factor IRF-2 exerts its oncogenic phenotype through the DNA binding/transcription repression domain. Oncogene, 11, 537–544. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

