Inter-strand photoproducts are produced in high yield within A-DNA exposed to UVC radiation
- PMID: 12799441
- PMCID: PMC162242
- DOI: 10.1093/nar/gkg408
Inter-strand photoproducts are produced in high yield within A-DNA exposed to UVC radiation
Abstract
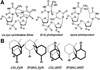
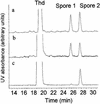
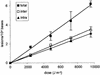
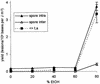
Far-UV irradiation of DNA leads to the dimerization of pyrimidine bases, resulting in the formation of cyclobutane type dimers and (6-4) photoproducts. In the dry state, an additional thymine dimeric photolesion, the spore photoproduct, is also generated. While most photoproducts are expected to be produced between adjacent pyrimidines, little attention has been paid to lesions involving bases located on different DNA strands. Using HPLC- mass spectrometry analysis of enzymatically digested DNA, we observed that, in the dry state, inter-strand dimeric photoproducts represented 30% of the total yield of dimeric thymine lesions. The major inter-strand damage was found to be the spore photoproduct. Formation of inter-strand lesions in significant yield could be obtained in solution upon modification of the DNA conformation as the result of the addition of large amounts of ethanol. In both cases, DNA is in the A-form, which is characterized by a high compaction, likely to favor inter-strand photoreactions. Since the latter DNA conformation is also predominant in bacterial spores, the formation and repair of dimeric photoproducts involving thymine bases located on different DNA strands may thus be relevant in terms of deleterious effects of UV radiation to the latter microorganisms.
Figures


Similar articles
-
Formation of the spore photoproduct and other dimeric lesions between adjacent pyrimidines in UVC-irradiated dry DNA.Photochem Photobiol Sci. 2003 Apr;2(4):433-6. doi: 10.1039/b300173c. Photochem Photobiol Sci. 2003. PMID: 12760543
-
Individual determination of the yield of the main UV-induced dimeric pyrimidine photoproducts in DNA suggests a high mutagenicity of CC photolesions.Biochemistry. 2001 Feb 27;40(8):2495-501. doi: 10.1021/bi0022543. Biochemistry. 2001. PMID: 11327871
-
Solar UV radiation-induced DNA Bipyrimidine photoproducts: formation and mechanistic insights.Top Curr Chem. 2015;356:249-75. doi: 10.1007/128_2014_553. Top Curr Chem. 2015. PMID: 25370518 Review.
-
UHPLC-Q-TOF/MS detection of UV-induced TpT dimeric lesions in genomic DNA.J Chromatogr B Analyt Technol Biomed Life Sci. 2018 Oct 1;1096:135-142. doi: 10.1016/j.jchromb.2018.08.015. Epub 2018 Aug 20. J Chromatogr B Analyt Technol Biomed Life Sci. 2018. PMID: 30170291
-
[Photochemical Processes of Cell DNA Damage by UV Radiation of Various Wavelengths: Biological Consequences].Mol Biol (Mosk). 2024 Jan-Feb;58(1):3-21. Mol Biol (Mosk). 2024. PMID: 38943577 Review. Russian.
Cited by
-
Evidence for Reverse Hoogsteen Hairpin Intermediates in the Photocrosslinking of Human Telomeric DNA Sequences.Photochem Photobiol. 2018 Jul;94(4):685-697. doi: 10.1111/php.12898. Epub 2018 Mar 31. Photochem Photobiol. 2018. PMID: 29418001 Free PMC article.
-
Spore photoproduct within DNA is a surprisingly poor substrate for its designated repair enzyme-The spore photoproduct lyase.DNA Repair (Amst). 2017 May;53:31-42. doi: 10.1016/j.dnarep.2016.11.005. Epub 2017 Mar 6. DNA Repair (Amst). 2017. PMID: 28320593 Free PMC article.
-
Kinetics of DNA Adducts and Abasic Site Formation in Tissues of Mice Treated with a Nitrogen Mustard.Chem Res Toxicol. 2020 Apr 20;33(4):988-998. doi: 10.1021/acs.chemrestox.0c00012. Epub 2020 Apr 2. Chem Res Toxicol. 2020. PMID: 32174110 Free PMC article.
-
Adaptation of DNA to Protein Binding Revealed by Spectroscopy and Molecular Simulation.J Phys Chem B. 2025 Jun 12;129(23):5653-5663. doi: 10.1021/acs.jpcb.5c00189. Epub 2025 May 28. J Phys Chem B. 2025. PMID: 40436634 Free PMC article.
-
UVC Irradiation for Pathogen Reduction of Platelet Concentrates and Plasma.Transfus Med Hemother. 2011;38(1):43-54. doi: 10.1159/000323845. Epub 2011 Jan 22. Transfus Med Hemother. 2011. PMID: 21779205 Free PMC article.
References
-
- Cadet J. and Vigny,P. (1990) Photochemistry of nucleic acids. In Morrison,H. (ed.), Bioorganic Photochemistry. Wiley, New York, NY, Vol. 1, pp. 1–272.
-
- Douki T. and Cadet,J. (1995) UV and nucleic acids. In Jornvall,H. and Jollès,P. (eds), Interface between Chemistry and Biochemistry. Birkhauser Verlag, Basel, Switzerland, pp. 173–197.
-
- Douki T. and Cadet,J. (2001) Individual determination of the yield of the main-UV induced dimeric pyrimidine photoproducts in DNA suggests a high mutagenicity of CC photolesions. Biochemistry, 40, 2495–2501. - PubMed
-
- Donnellan J.E. and Setlow,R.B. (1965) Thymine photoproducts but not thymine dimers found in ultraviolet-irradiated bacterial spores. Science, 149, 308–310. - PubMed
-
- Varghese A.J. (1970) 5-Thyminyl-5,6-dihydrothymine from DNA irradiated with ultraviolet light. Biochem. Biophys. Res. Commun., 38, 484–490. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

