Triplex formation with 2'-O,4'-C-ethylene-bridged nucleic acids (ENA) having C3'-endo conformation at physiological pH
- PMID: 12799454
- PMCID: PMC162250
- DOI: 10.1093/nar/gkg416
Triplex formation with 2'-O,4'-C-ethylene-bridged nucleic acids (ENA) having C3'-endo conformation at physiological pH
Abstract
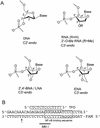
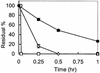
Antigenes, which are substances that inhibit gene expression by binding to double-stranded DNA (dsDNA) in a sequence-specific manner, are currently sought for the treatment of various gene-related diseases. As such antigenes, we developed new nuclease-resistant oligopyrimidine nucleotides that are partially modified with 2'-O,4'-C-ethylene nucleic acids (ENA), which are constrained in the C3'-endo conformation and can form a triplex with dsDNA at physiological pH. It was found that these oligonucleotides formed triplexes similarly to those partially modified with 2'-O,4'-C-methylene nucleic acids (2',4'-BNA or LNA), as determined by UV melting analyses, electromobility shift assays, CD spectral analyses and restriction enzyme inhibition assays. In our studies, oligonucleotides fully modified with ENA have delta torsion angle values that are marginally higher than those of 2',4'-BNA/LNA. ENA oligonucleotides present in 10-fold the amount of dsDNA were found to be favorable in forming triplexes. These results provide useful information for the future design of triplex-forming oligonucleotides fully modified with such nucleic acids constrained in the C3'-endo conformation considering that oligonucleotides fully modified with 2',4'-BNA/LNA do not form triplexes.
Figures



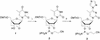
Similar articles
-
2'-O,4'-C-ethylene-bridged nucleic acids (ENA) with nuclease-resistance and high affinity for RNA.Nucleic Acids Res Suppl. 2001;(1):241-2. doi: 10.1093/nass/1.1.241. Nucleic Acids Res Suppl. 2001. PMID: 12836354
-
Synthesis and properties of 2'-O,4'-C-ethylene-bridged nucleic acids (ENA) as effective antisense oligonucleotides.Bioorg Med Chem. 2003 May 15;11(10):2211-26. doi: 10.1016/s0968-0896(03)00115-9. Bioorg Med Chem. 2003. PMID: 12713831
-
2'-O,4'-C-Methylene bridged nucleic acid (2',4'-BNA): synthesis and triplex-forming properties.Bioorg Med Chem. 2001 Apr;9(4):1001-11. doi: 10.1016/s0968-0896(00)00325-4. Bioorg Med Chem. 2001. PMID: 11354656
-
2'-O,4'-C-ethylene-bridged nucleic acids (ENA) as next-generation antisense and antigene agents.Biol Pharm Bull. 2004 Apr;27(4):453-6. doi: 10.1248/bpb.27.453. Biol Pharm Bull. 2004. PMID: 15056846 Review.
-
ENA oligonucleotides as therapeutics.Curr Opin Mol Ther. 2006 Apr;8(2):144-9. Curr Opin Mol Ther. 2006. PMID: 16610767 Review.
Cited by
-
Allele-selective inhibition of mutant huntingtin expression with antisense oligonucleotides targeting the expanded CAG repeat.Biochemistry. 2010 Nov 30;49(47):10166-78. doi: 10.1021/bi101208k. Epub 2010 Nov 8. Biochemistry. 2010. PMID: 21028906 Free PMC article.
-
Successful skipping of abnormal pseudoexon by antisense oligonucleotides in vitro for a patient with beta-propeller protein-associated neurodegeneration.Sci Rep. 2024 Mar 18;14(1):6506. doi: 10.1038/s41598-024-56704-z. Sci Rep. 2024. PMID: 38499569 Free PMC article.
-
Solution structure of a dsDNA:LNA triplex.Nucleic Acids Res. 2004 Nov 18;32(20):6078-85. doi: 10.1093/nar/gkh942. Print 2004. Nucleic Acids Res. 2004. PMID: 15550567 Free PMC article.
-
Optimized DNA-targeting using triplex forming C5-alkynyl functionalized LNA.Chem Commun (Camb). 2009 Nov 28;(44):6756-8. doi: 10.1039/b917312a. Epub 2009 Oct 12. Chem Commun (Camb). 2009. PMID: 19885469 Free PMC article.
-
Locked Nucleic Acid Oligonucleotides Facilitate RNA•LNA-RNA Triple-Helix Formation and Reduce MALAT1 Levels.Int J Mol Sci. 2024 Jan 28;25(3):1630. doi: 10.3390/ijms25031630. Int J Mol Sci. 2024. PMID: 38338910 Free PMC article.
References
-
- Moser H.E. and Dervan,P.B. (1987) Sequence-specific cleavage of double helical DNA by triple helix formation. Science, 238, 645–650. - PubMed
-
- Le Doan T., Perrouault,L., Praseuth,D., Habhoub,N., Decout,J.L., Thuong,N.T., Lhomme,J. and Hélène,C. (1987) Sequence-specific recognition, photocrosslinking and cleavage of the DNA double helix by an oligo-[α]-thymidylate covalently linked to an azidoproflavine derivative. Nucleic Acids Res., 15, 7749–7760. - PMC - PubMed
-
- Povsic T.J. and Dervan,P.B. (1989) Triple helix formation by oligonucleotides on DNA extended to the physiological pH range. J. Am. Chem. Soc., 111, 3059–3061.
-
- Obika S., Uneda,T., Sugimoto,T., Nanbu,D., Minami,T., Doi,T. and Imanishi,T. (2001) 2′-O,4′-C-Methylene bridged nucleic acid (2′,4′-BNA): synthesis and triplex-forming properties. Bioorg. Med. Chem., 9, 1001–1011. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

